| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229774 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2016 | 6 Pages |
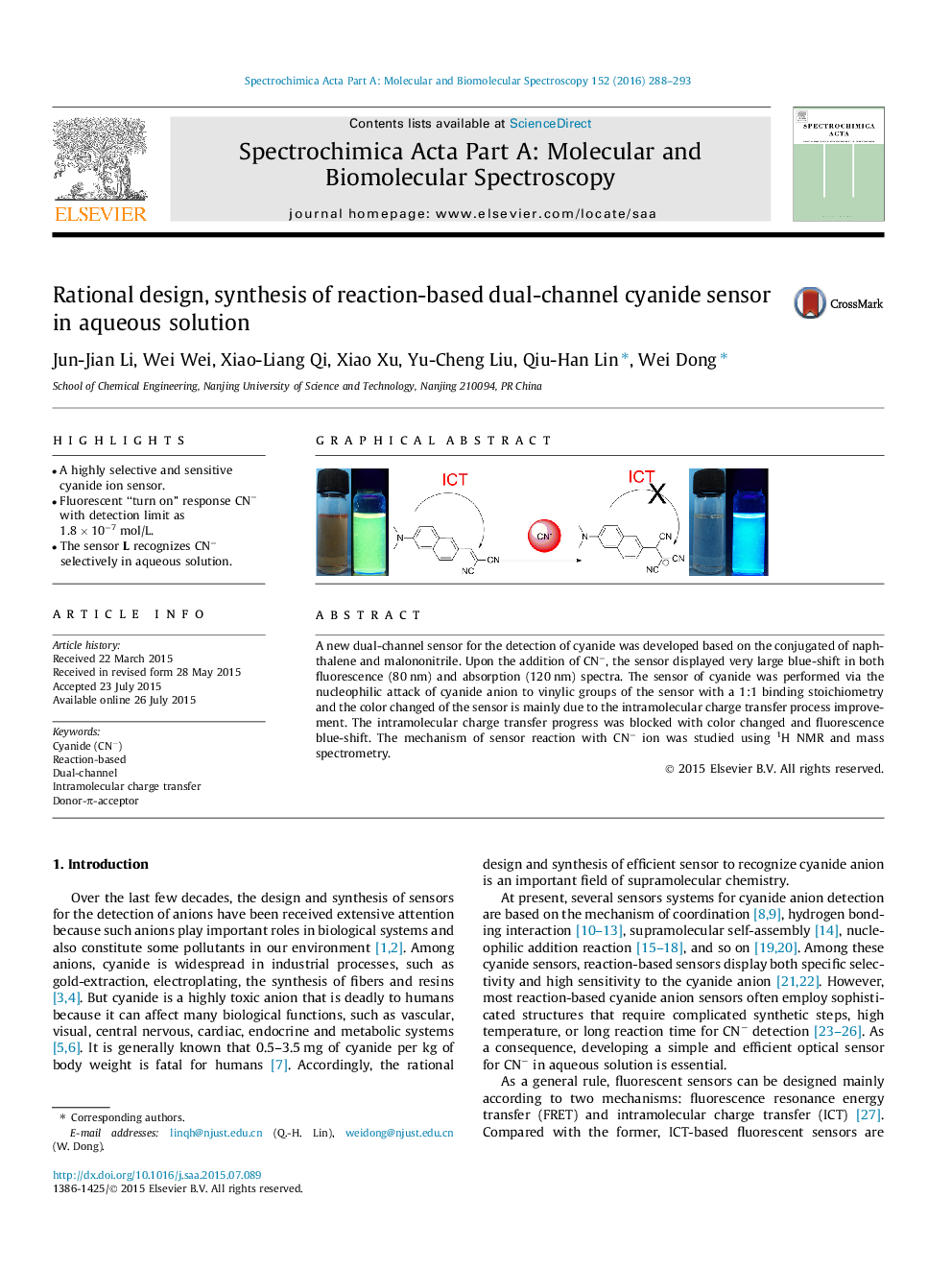
•A highly selective and sensitive cyanide ion sensor.•Fluorescent “turn on” response CN− with detection limit as 1.8 × 10−7 mol/L.•The sensor L recognizes CN− selectively in aqueous solution.
A new dual-channel sensor for the detection of cyanide was developed based on the conjugated of naphthalene and malononitrile. Upon the addition of CN−, the sensor displayed very large blue-shift in both fluorescence (80 nm) and absorption (120 nm) spectra. The sensor of cyanide was performed via the nucleophilic attack of cyanide anion to vinylic groups of the sensor with a 1:1 binding stoichiometry and the color changed of the sensor is mainly due to the intramolecular charge transfer process improvement. The intramolecular charge transfer progress was blocked with color changed and fluorescence blue-shift. The mechanism of sensor reaction with CN− ion was studied using 1H NMR and mass spectrometry.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide