| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229784 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2016 | 8 Pages |
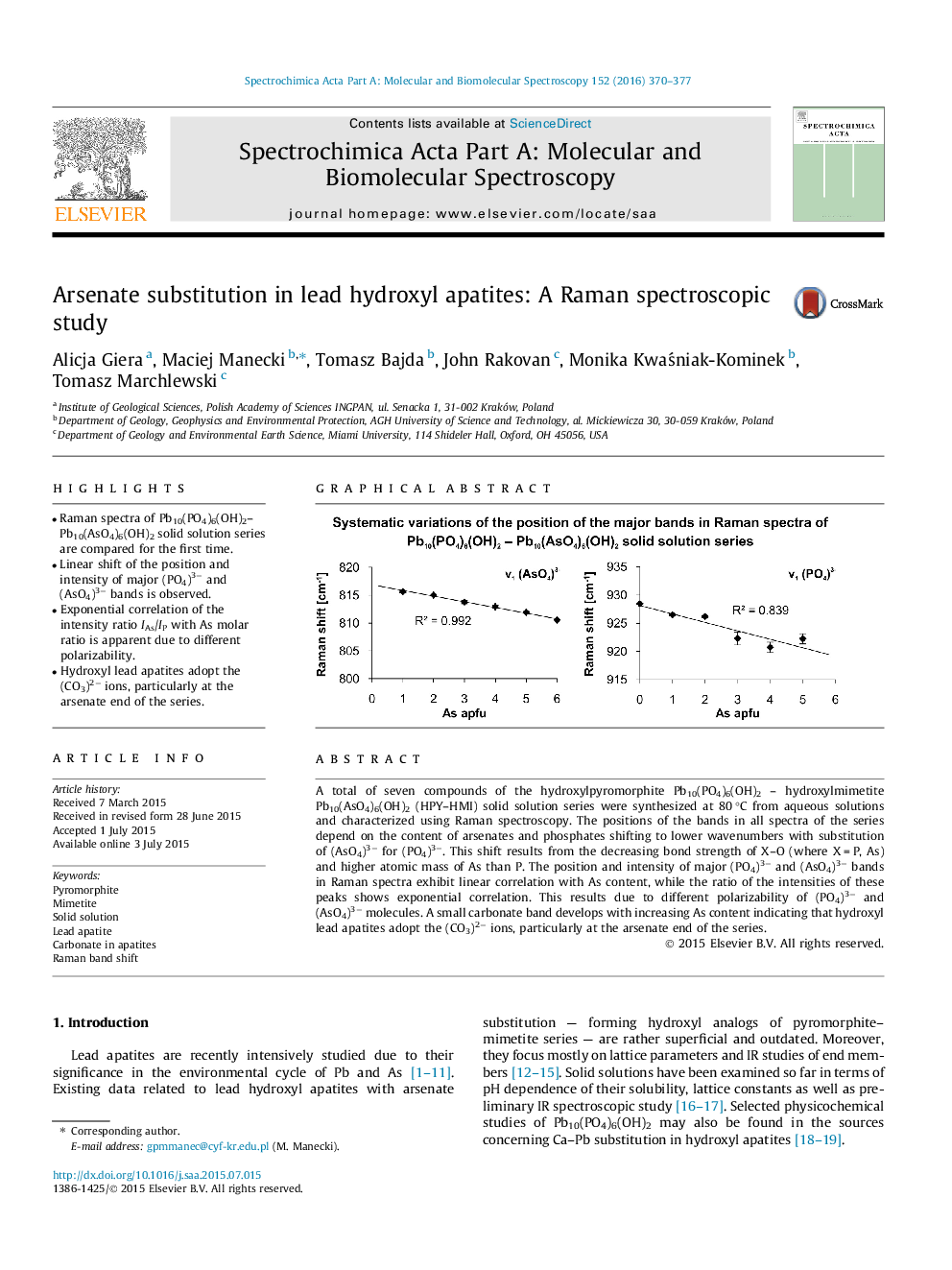
•Raman spectra of Pb10(PO4)6(OH)2–Pb10(AsO4)6(OH)2 solid solution series are compared for the first time.•Linear shift of the position and intensity of major (PO4)3− and (AsO4)3− bands is observed.•Exponential correlation of the intensity ratio IAs/IP with As molar ratio is apparent due to different polarizability.•Hydroxyl lead apatites adopt the (CO3)2− ions, particularly at the arsenate end of the series.
A total of seven compounds of the hydroxylpyromorphite Pb10(PO4)6(OH)2 – hydroxylmimetite Pb10(AsO4)6(OH)2 (HPY–HMI) solid solution series were synthesized at 80 °C from aqueous solutions and characterized using Raman spectroscopy. The positions of the bands in all spectra of the series depend on the content of arsenates and phosphates shifting to lower wavenumbers with substitution of (AsO4)3− for (PO4)3−. This shift results from the decreasing bond strength of X–O (where X = P, As) and higher atomic mass of As than P. The position and intensity of major (PO4)3− and (AsO4)3− bands in Raman spectra exhibit linear correlation with As content, while the ratio of the intensities of these peaks shows exponential correlation. This results due to different polarizability of (PO4)3− and (AsO4)3− molecules. A small carbonate band develops with increasing As content indicating that hydroxyl lead apatites adopt the (CO3)2− ions, particularly at the arsenate end of the series.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide