| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229858 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 11 Pages |
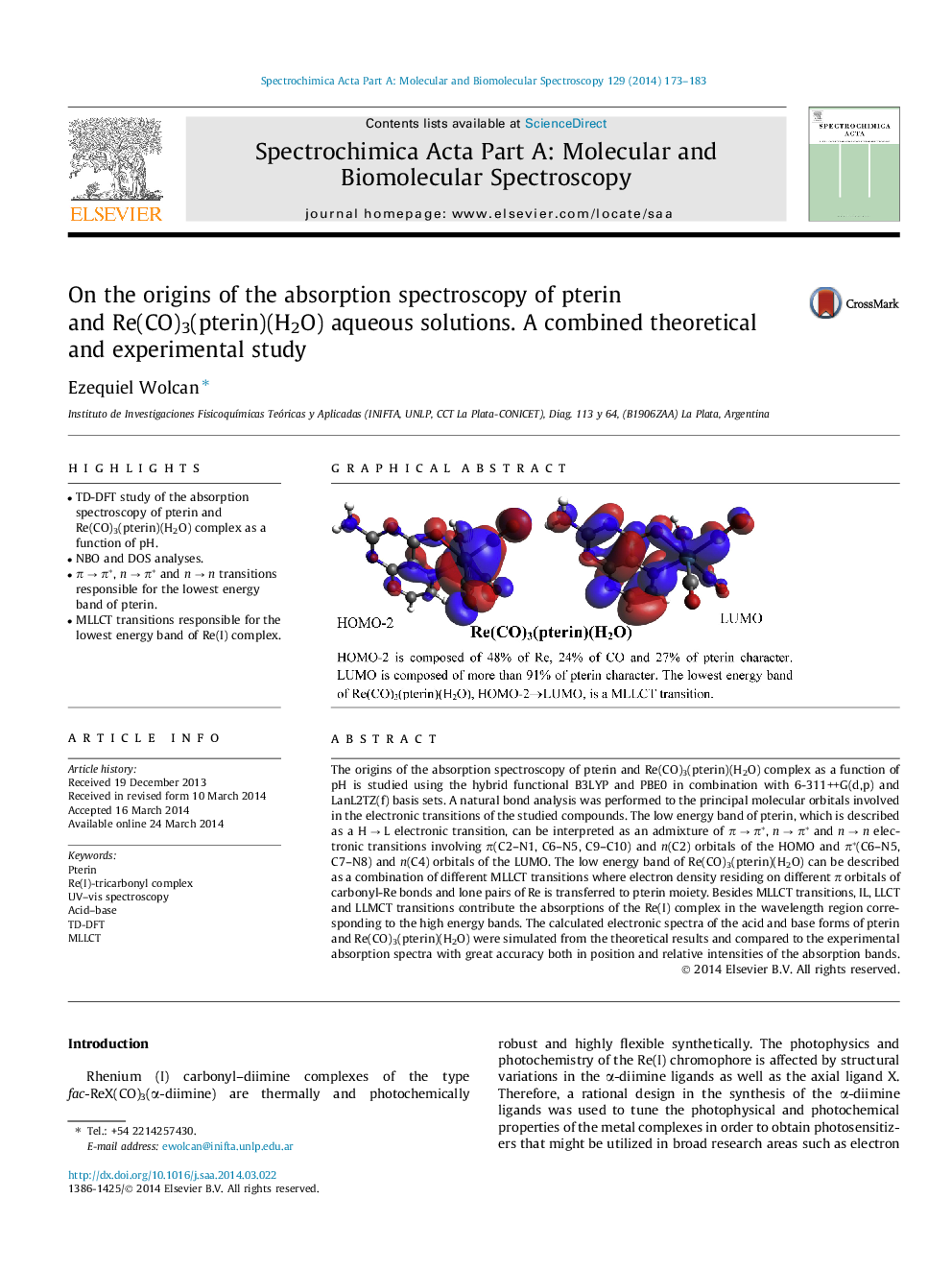
•TD-DFT study of the absorption spectroscopy of pterin and Re(CO)3(pterin)(H2O) complex as a function of pH.•NBO and DOS analyses.•π → π*, n → π* and n → n transitions responsible for the lowest energy band of pterin.•MLLCT transitions responsible for the lowest energy band of Re(I) complex.
The origins of the absorption spectroscopy of pterin and Re(CO)3(pterin)(H2O) complex as a function of pH is studied using the hybrid functional B3LYP and PBE0 in combination with 6-311++G(d,p) and LanL2TZ(f) basis sets. A natural bond analysis was performed to the principal molecular orbitals involved in the electronic transitions of the studied compounds. The low energy band of pterin, which is described as a H → L electronic transition, can be interpreted as an admixture of π → π*, n → π* and n → n electronic transitions involving π(C2–N1, C6–N5, C9–C10) and n(C2) orbitals of the HOMO and π*(C6–N5, C7–N8) and n(C4) orbitals of the LUMO. The low energy band of Re(CO)3(pterin)(H2O) can be described as a combination of different MLLCT transitions where electron density residing on different π orbitals of carbonyl-Re bonds and lone pairs of Re is transferred to pterin moiety. Besides MLLCT transitions, IL, LLCT and LLMCT transitions contribute the absorptions of the Re(I) complex in the wavelength region corresponding to the high energy bands. The calculated electronic spectra of the acid and base forms of pterin and Re(CO)3(pterin)(H2O) were simulated from the theoretical results and compared to the experimental absorption spectra with great accuracy both in position and relative intensities of the absorption bands.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide