| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229914 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 11 Pages |
•Synthesis of three new Mn(II) complexes with N,O-donor heteroaromatic alcohol and aldehyde.•Compounds were characterised by X-ray, IR, Raman, EPR with DFT, magnetic and thermal studies.•Mn(II) ion is eight-coordinated in 1 and 2, whereas in 3 the coordination number of Mn(II) is six.•The ligands and the Mn(II) complexes have been tested in vitro against Gram(+) and Gram(−) bacteria.•Antibacterial activity of the Mn(II) complexes is selective for Gram(+) or Gram(−) bacteria.
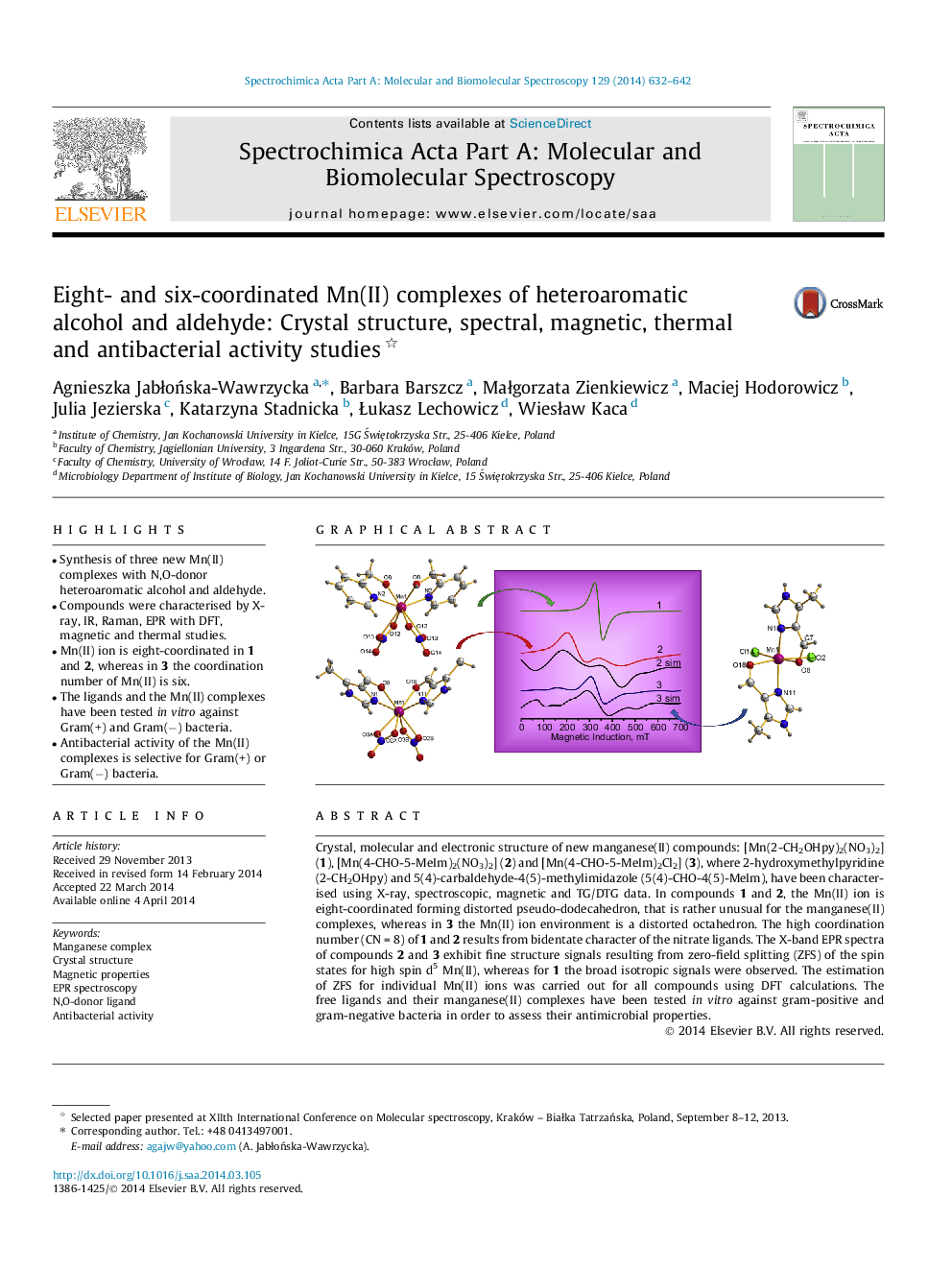
Crystal, molecular and electronic structure of new manganese(II) compounds: [Mn(2-CH2OHpy)2(NO3)2] (1), [Mn(4-CHO-5-MeIm)2(NO3)2] (2) and [Mn(4-CHO-5-MeIm)2Cl2] (3), where 2-hydroxymethylpyridine (2-CH2OHpy) and 5(4)-carbaldehyde-4(5)-methylimidazole (5(4)-CHO-4(5)-MeIm), have been characterised using X-ray, spectroscopic, magnetic and TG/DTG data. In compounds 1 and 2, the Mn(II) ion is eight-coordinated forming distorted pseudo-dodecahedron, that is rather unusual for the manganese(II) complexes, whereas in 3 the Mn(II) ion environment is a distorted octahedron. The high coordination number (CN = 8) of 1 and 2 results from bidentate character of the nitrate ligands. The X-band EPR spectra of compounds 2 and 3 exhibit fine structure signals resulting from zero-field splitting (ZFS) of the spin states for high spin d5 Mn(II), whereas for 1 the broad isotropic signals were observed. The estimation of ZFS for individual Mn(II) ions was carried out for all compounds using DFT calculations. The free ligands and their manganese(II) complexes have been tested in vitro against gram-positive and gram-negative bacteria in order to assess their antimicrobial properties.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide