| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229921 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
•Distinct spherical Ag nanoparticles were prepared by chemical reduction method.•The reduction process and nucleation stage were enhanced by the parameters.•Nanoparticles shape, size and distribution are influenced by the parameters.
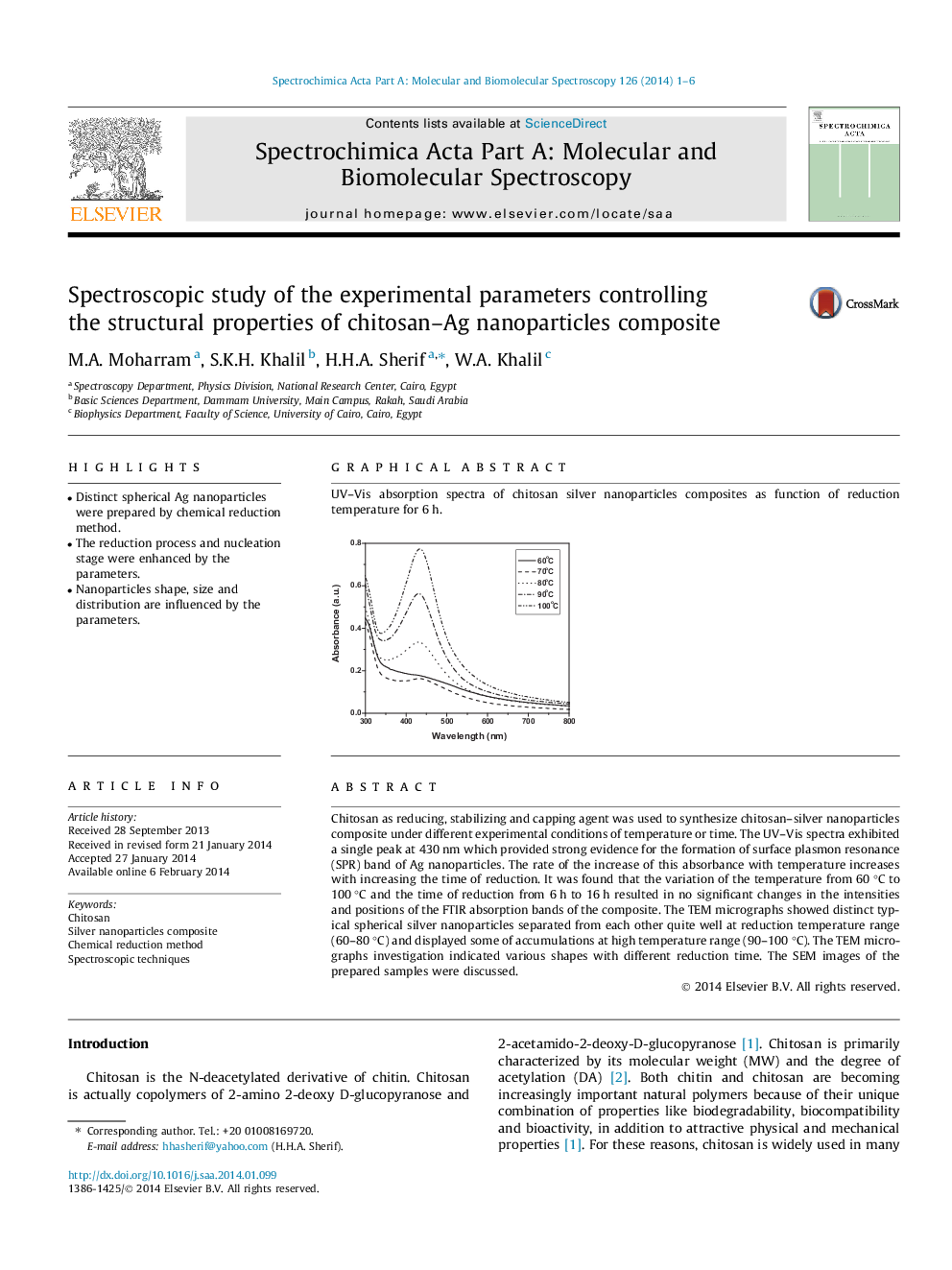
Chitosan as reducing, stabilizing and capping agent was used to synthesize chitosan–silver nanoparticles composite under different experimental conditions of temperature or time. The UV–Vis spectra exhibited a single peak at 430 nm which provided strong evidence for the formation of surface plasmon resonance (SPR) band of Ag nanoparticles. The rate of the increase of this absorbance with temperature increases with increasing the time of reduction. It was found that the variation of the temperature from 60 °C to 100 °C and the time of reduction from 6 h to 16 h resulted in no significant changes in the intensities and positions of the FTIR absorption bands of the composite. The TEM micrographs showed distinct typical spherical silver nanoparticles separated from each other quite well at reduction temperature range (60–80 °C) and displayed some of accumulations at high temperature range (90–100 °C). The TEM micrographs investigation indicated various shapes with different reduction time. The SEM images of the prepared samples were discussed.
Graphical abstractUV–Vis absorption spectra of chitosan silver nanoparticles composites as function of reduction temperature for 6 h.Figure optionsDownload full-size imageDownload as PowerPoint slide