| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229930 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 8 Pages |
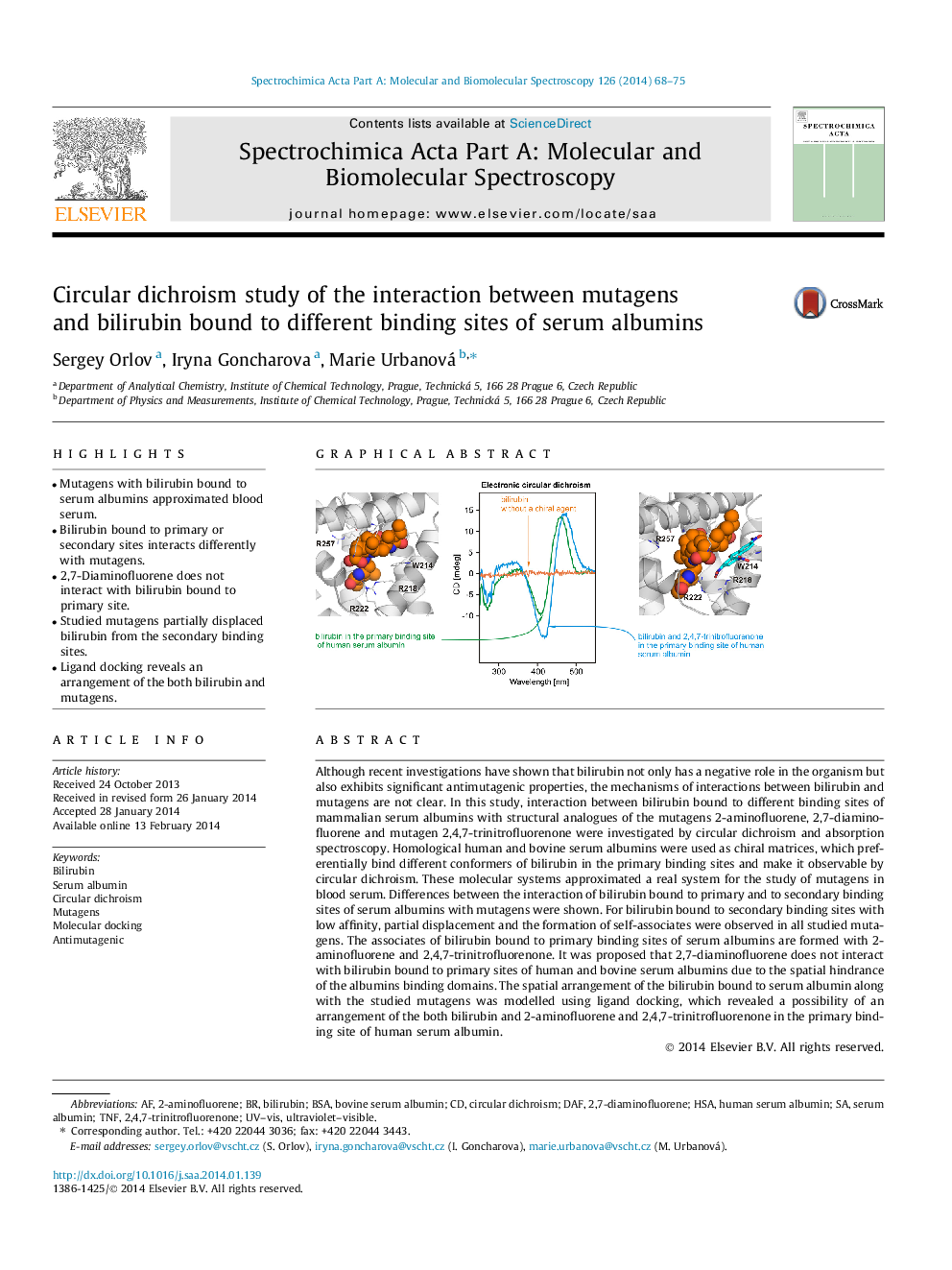
•Mutagens with bilirubin bound to serum albumins approximated blood serum.•Bilirubin bound to primary or secondary sites interacts differently with mutagens.•2,7-Diaminofluorene does not interact with bilirubin bound to primary site.•Studied mutagens partially displaced bilirubin from the secondary binding sites.•Ligand docking reveals an arrangement of the both bilirubin and mutagens.
Although recent investigations have shown that bilirubin not only has a negative role in the organism but also exhibits significant antimutagenic properties, the mechanisms of interactions between bilirubin and mutagens are not clear. In this study, interaction between bilirubin bound to different binding sites of mammalian serum albumins with structural analogues of the mutagens 2-aminofluorene, 2,7-diaminofluorene and mutagen 2,4,7-trinitrofluorenone were investigated by circular dichroism and absorption spectroscopy. Homological human and bovine serum albumins were used as chiral matrices, which preferentially bind different conformers of bilirubin in the primary binding sites and make it observable by circular dichroism. These molecular systems approximated a real system for the study of mutagens in blood serum. Differences between the interaction of bilirubin bound to primary and to secondary binding sites of serum albumins with mutagens were shown. For bilirubin bound to secondary binding sites with low affinity, partial displacement and the formation of self-associates were observed in all studied mutagens. The associates of bilirubin bound to primary binding sites of serum albumins are formed with 2-aminofluorene and 2,4,7-trinitrofluorenone. It was proposed that 2,7-diaminofluorene does not interact with bilirubin bound to primary sites of human and bovine serum albumins due to the spatial hindrance of the albumins binding domains. The spatial arrangement of the bilirubin bound to serum albumin along with the studied mutagens was modelled using ligand docking, which revealed a possibility of an arrangement of the both bilirubin and 2-aminofluorene and 2,4,7-trinitrofluorenone in the primary binding site of human serum albumin.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide