| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229935 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 7 Pages |
•Nine novel bis-azo dyes were synthesized and fully characterized.•Spectroscopic properties were examined by UV–vis, FT-IR and NMR techniques.•The azo-hydrazone tautomeric forms of prepared dyes were investigated.•Effects of pH on the absorption spectrum of dyes were studied.
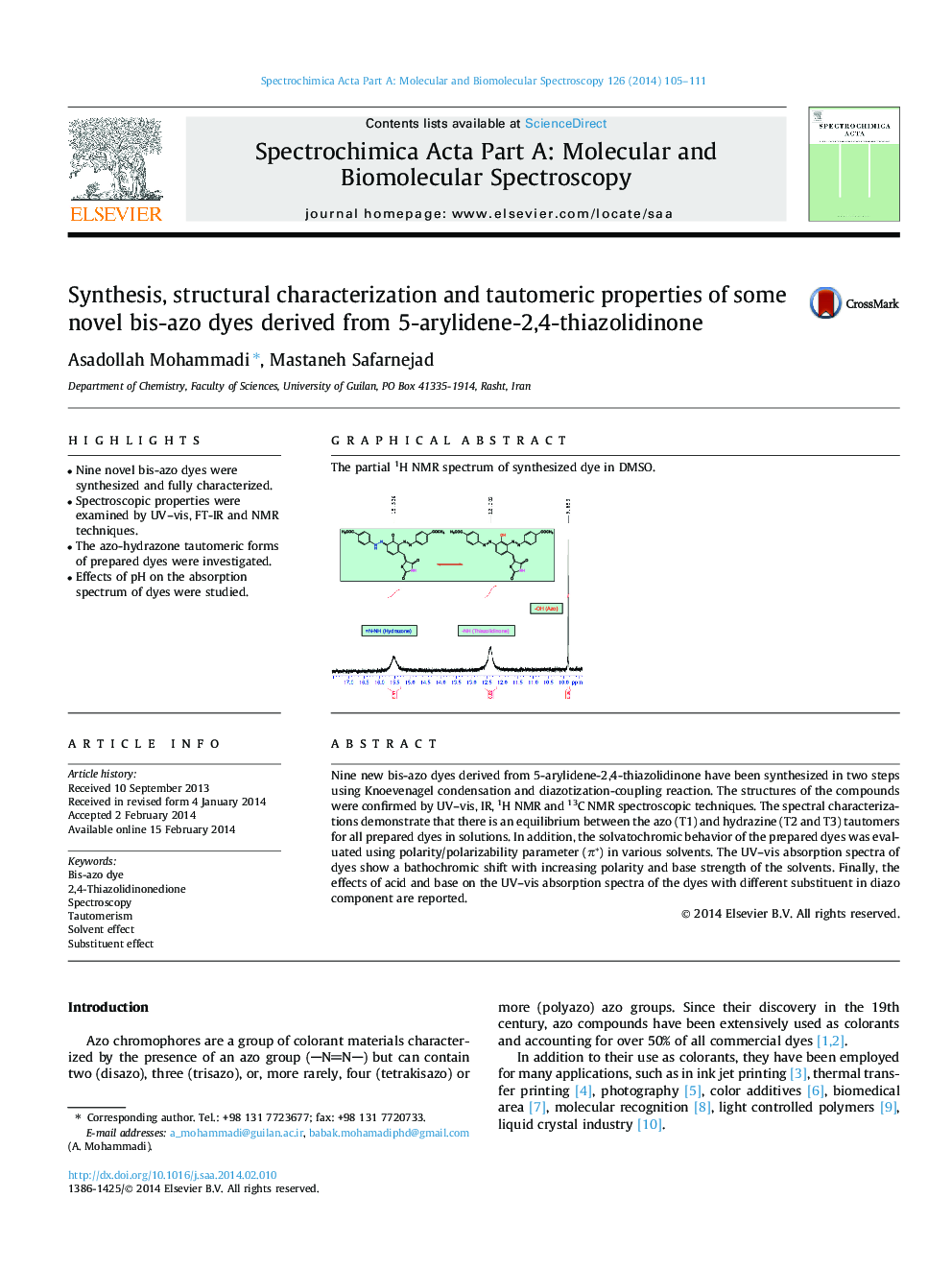
Nine new bis-azo dyes derived from 5-arylidene-2,4-thiazolidinone have been synthesized in two steps using Knoevenagel condensation and diazotization-coupling reaction. The structures of the compounds were confirmed by UV–vis, IR, 1H NMR and 13C NMR spectroscopic techniques. The spectral characterizations demonstrate that there is an equilibrium between the azo (T1) and hydrazine (T2 and T3) tautomers for all prepared dyes in solutions. In addition, the solvatochromic behavior of the prepared dyes was evaluated using polarity/polarizability parameter (π*) in various solvents. The UV–vis absorption spectra of dyes show a bathochromic shift with increasing polarity and base strength of the solvents. Finally, the effects of acid and base on the UV–vis absorption spectra of the dyes with different substituent in diazo component are reported.
Graphical abstractThe partial 1H NMR spectrum of synthesized dye in DMSO.Figure optionsDownload full-size imageDownload as PowerPoint slide