| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229957 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 10 Pages |
•Removing a mixture of alizarin red and alizarin yellow from aqueous solution.•Introducing a new and efficient adsorbent called nano γ-alumina.•Developing Taguchi design for multivariate optimization of this adsorption system.•Optimization of removal percent and capacity uptake using principle component analysis (PCA) followed by Taguchi method.•Isotherms modeling and kinetic studies of the current adsorption system.
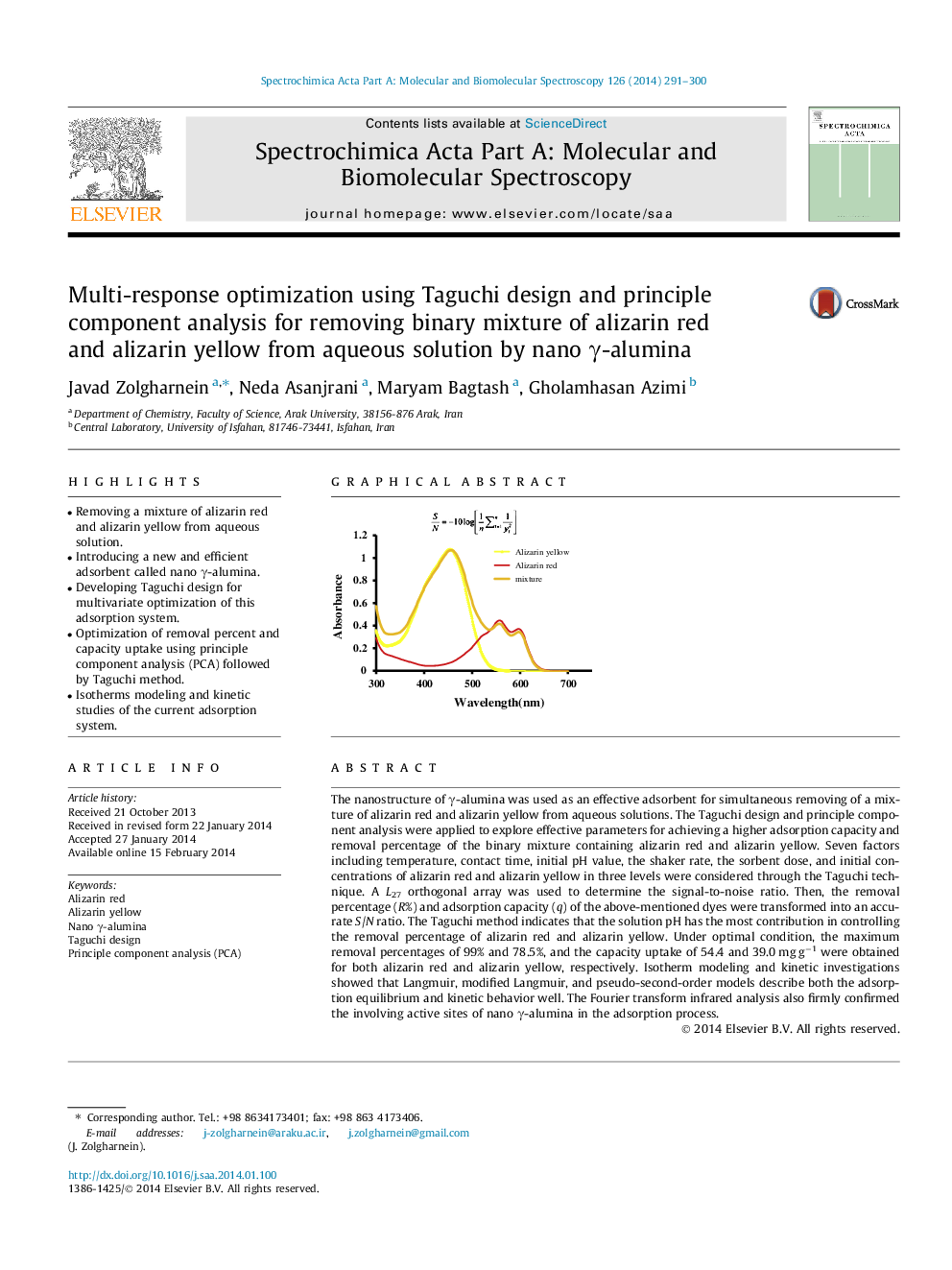
The nanostructure of γ-alumina was used as an effective adsorbent for simultaneous removing of a mixture of alizarin red and alizarin yellow from aqueous solutions. The Taguchi design and principle component analysis were applied to explore effective parameters for achieving a higher adsorption capacity and removal percentage of the binary mixture containing alizarin red and alizarin yellow. Seven factors including temperature, contact time, initial pH value, the shaker rate, the sorbent dose, and initial concentrations of alizarin red and alizarin yellow in three levels were considered through the Taguchi technique. A L27 orthogonal array was used to determine the signal-to-noise ratio. Then, the removal percentage (R%) and adsorption capacity (q) of the above-mentioned dyes were transformed into an accurate S/N ratio. The Taguchi method indicates that the solution pH has the most contribution in controlling the removal percentage of alizarin red and alizarin yellow. Under optimal condition, the maximum removal percentages of 99% and 78.5%, and the capacity uptake of 54.4 and 39.0 mg g−1 were obtained for both alizarin red and alizarin yellow, respectively. Isotherm modeling and kinetic investigations showed that Langmuir, modified Langmuir, and pseudo-second-order models describe both the adsorption equilibrium and kinetic behavior well. The Fourier transform infrared analysis also firmly confirmed the involving active sites of nano γ-alumina in the adsorption process.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide