| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230046 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 5 Pages |
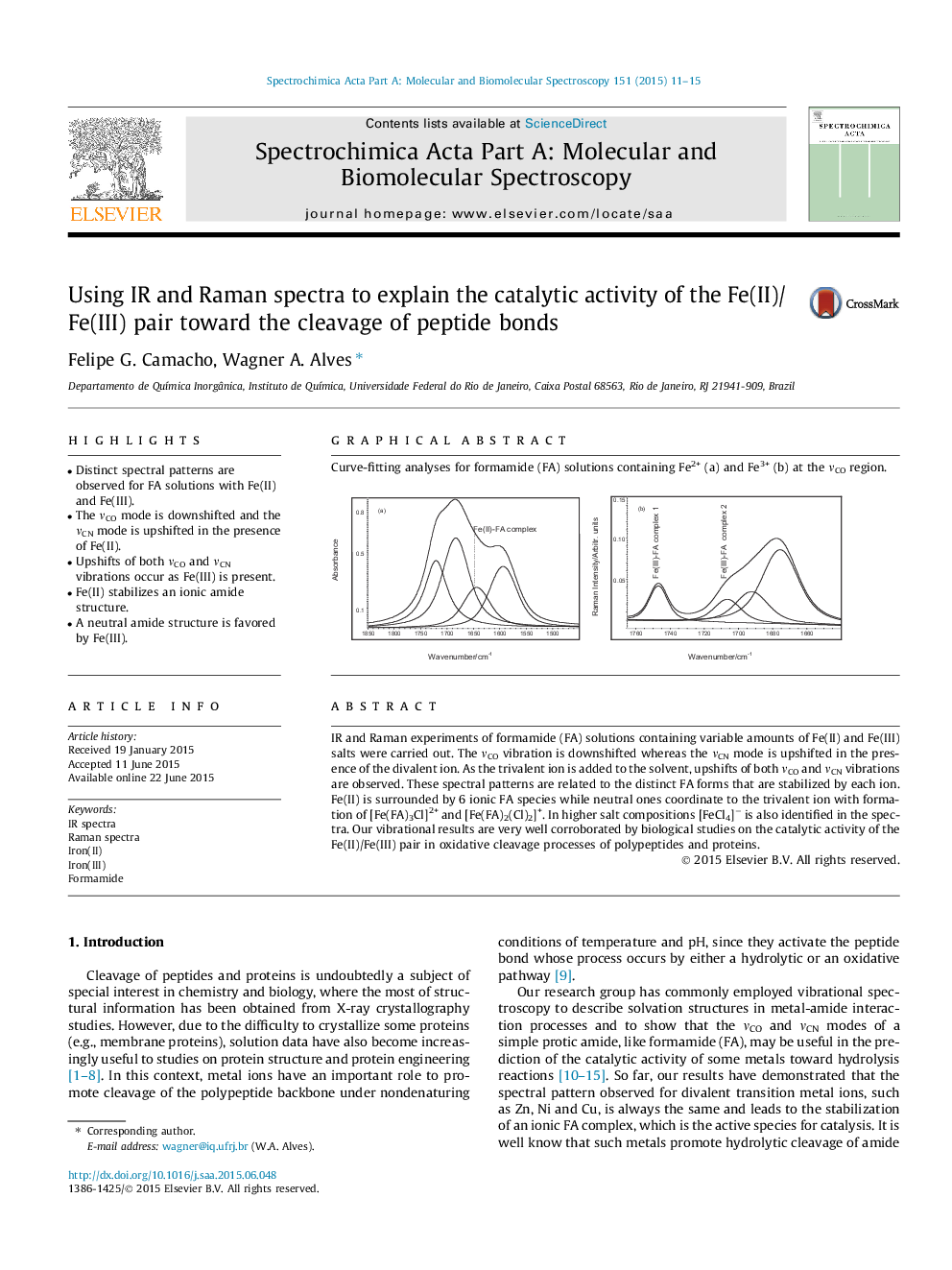
•Distinct spectral patterns are observed for FA solutions with Fe(II) and Fe(III).•The νCO mode is downshifted and the νCN mode is upshifted in the presence of Fe(II).•Upshifts of both νCO and νCN vibrations occur as Fe(III) is present.•Fe(II) stabilizes an ionic amide structure.•A neutral amide structure is favored by Fe(III).
IR and Raman experiments of formamide (FA) solutions containing variable amounts of Fe(II) and Fe(III) salts were carried out. The νCO vibration is downshifted whereas the νCN mode is upshifted in the presence of the divalent ion. As the trivalent ion is added to the solvent, upshifts of both νCO and νCN vibrations are observed. These spectral patterns are related to the distinct FA forms that are stabilized by each ion. Fe(II) is surrounded by 6 ionic FA species while neutral ones coordinate to the trivalent ion with formation of [Fe(FA)3Cl]2+ and [Fe(FA)2(Cl)2]+. In higher salt compositions [FeCl4]− is also identified in the spectra. Our vibrational results are very well corroborated by biological studies on the catalytic activity of the Fe(II)/Fe(III) pair in oxidative cleavage processes of polypeptides and proteins.
Graphical abstractCurve-fitting analyses for formamide (FA) solutions containing Fe2+ (a) and Fe3+ (b) at the νCO region.Figure optionsDownload full-size imageDownload as PowerPoint slide