| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230073 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 7 Pages |
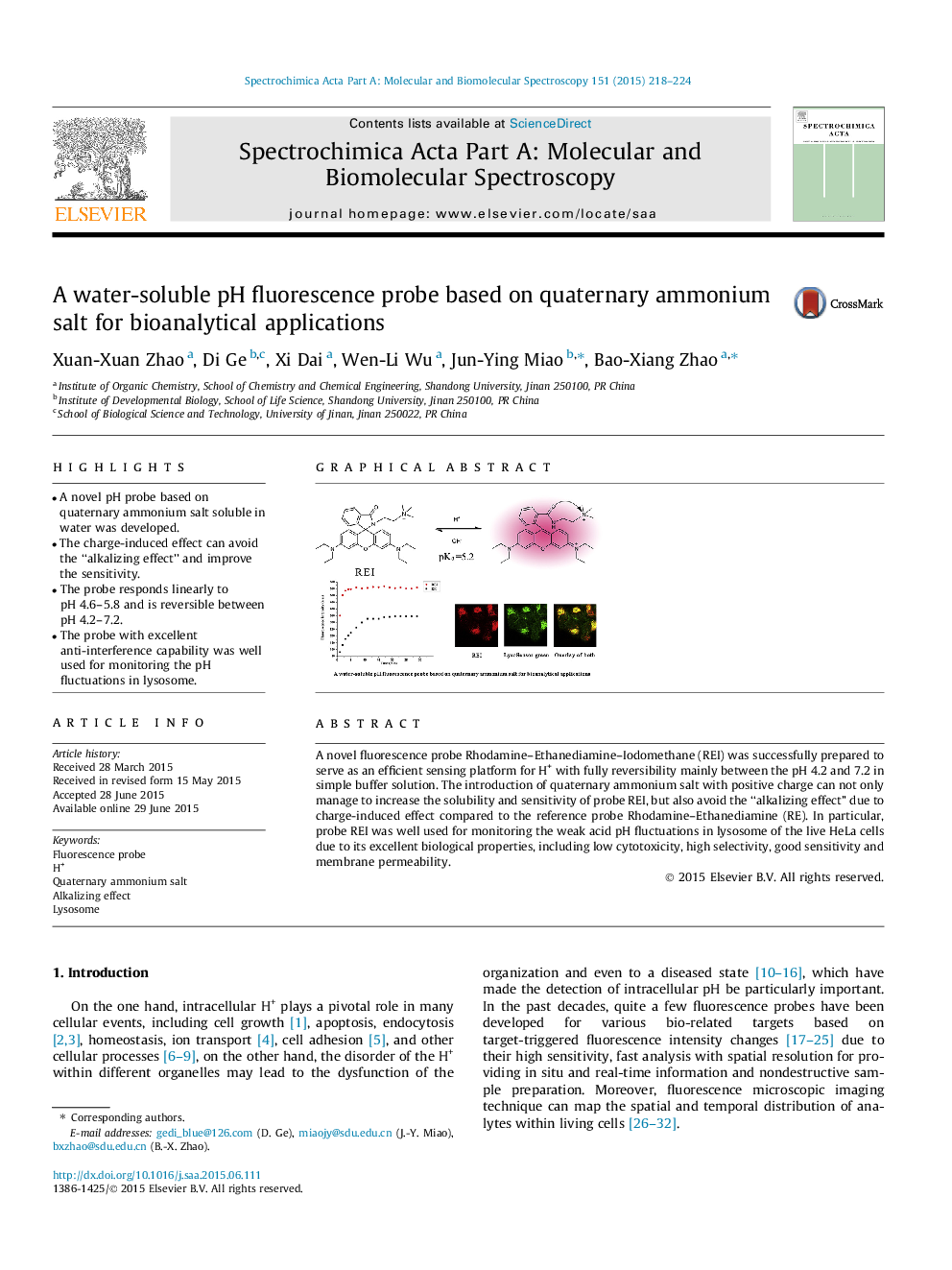
•A novel pH probe based on quaternary ammonium salt soluble in water was developed.•The charge-induced effect can avoid the “alkalizing effect” and improve the sensitivity.•The probe responds linearly to pH 4.6–5.8 and is reversible between pH 4.2–7.2.•The probe with excellent anti-interference capability was well used for monitoring the pH fluctuations in lysosome.
A novel fluorescence probe Rhodamine–Ethanediamine–Iodomethane (REI) was successfully prepared to serve as an efficient sensing platform for H+ with fully reversibility mainly between the pH 4.2 and 7.2 in simple buffer solution. The introduction of quaternary ammonium salt with positive charge can not only manage to increase the solubility and sensitivity of probe REI, but also avoid the “alkalizing effect” due to charge-induced effect compared to the reference probe Rhodamine–Ethanediamine (RE). In particular, probe REI was well used for monitoring the weak acid pH fluctuations in lysosome of the live HeLa cells due to its excellent biological properties, including low cytotoxicity, high selectivity, good sensitivity and membrane permeability.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide