| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230086 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 13 Pages |
•Synthesis of some azo pyridine derivatives.•Studying the molecular and electronic structures.•Studying the CT-DNA binding of the compounds.•Studying the catalytic oxidation of benzyl alcohol.
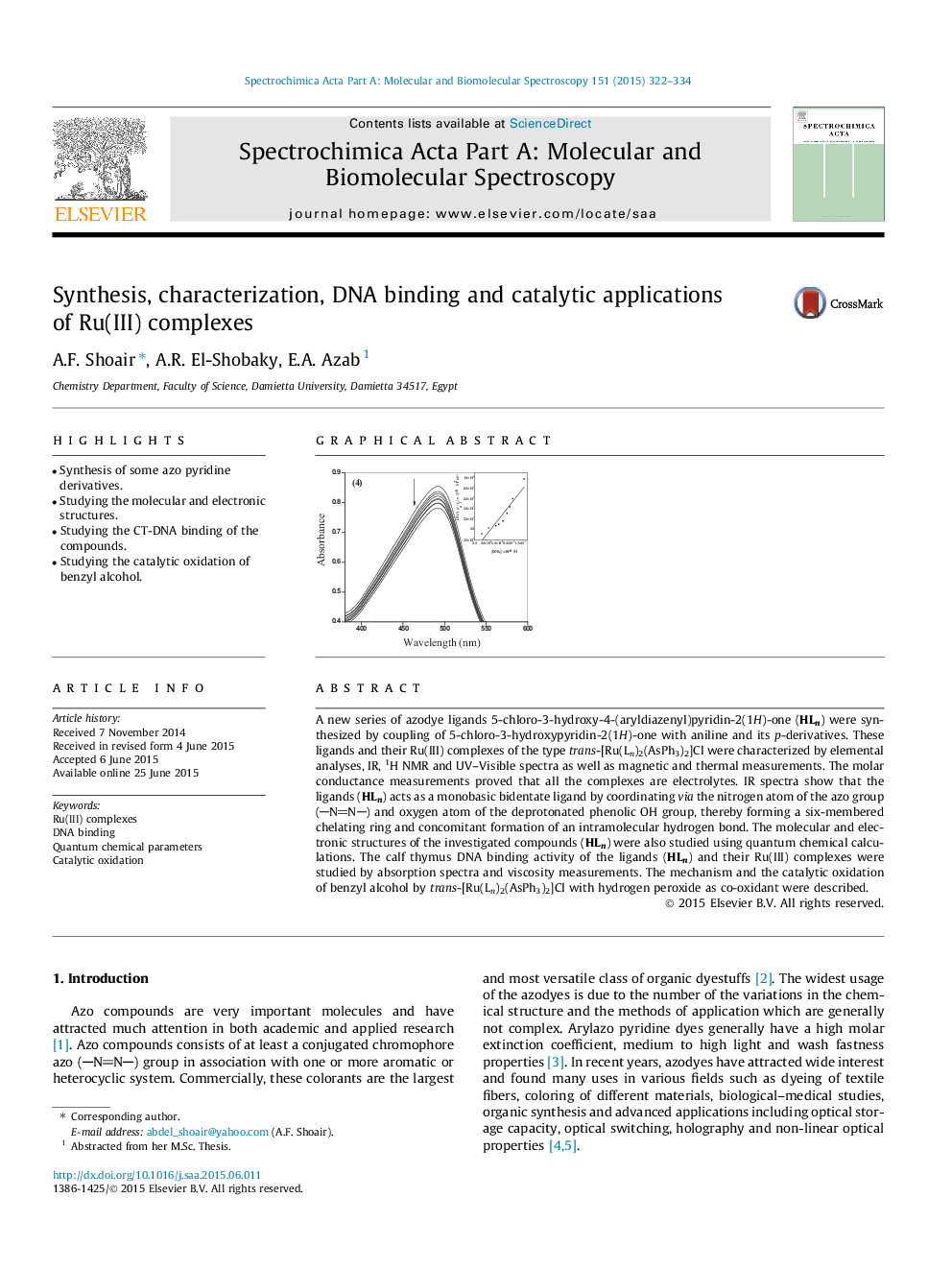
A new series of azodye ligands 5-chloro-3-hydroxy-4-(aryldiazenyl)pyridin-2(1H)-one (HLn) were synthesized by coupling of 5-chloro-3-hydroxypyridin-2(1H)-one with aniline and its p-derivatives. These ligands and their Ru(III) complexes of the type trans-[Ru(Ln)2(AsPh3)2]Cl were characterized by elemental analyses, IR, 1H NMR and UV–Visible spectra as well as magnetic and thermal measurements. The molar conductance measurements proved that all the complexes are electrolytes. IR spectra show that the ligands (HLn) acts as a monobasic bidentate ligand by coordinating via the nitrogen atom of the azo group (NN) and oxygen atom of the deprotonated phenolic OH group, thereby forming a six-membered chelating ring and concomitant formation of an intramolecular hydrogen bond. The molecular and electronic structures of the investigated compounds (HLn) were also studied using quantum chemical calculations. The calf thymus DNA binding activity of the ligands (HLn) and their Ru(III) complexes were studied by absorption spectra and viscosity measurements. The mechanism and the catalytic oxidation of benzyl alcohol by trans-[Ru(Ln)2(AsPh3)2]Cl with hydrogen peroxide as co-oxidant were described.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide