| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230107 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 8 Pages |
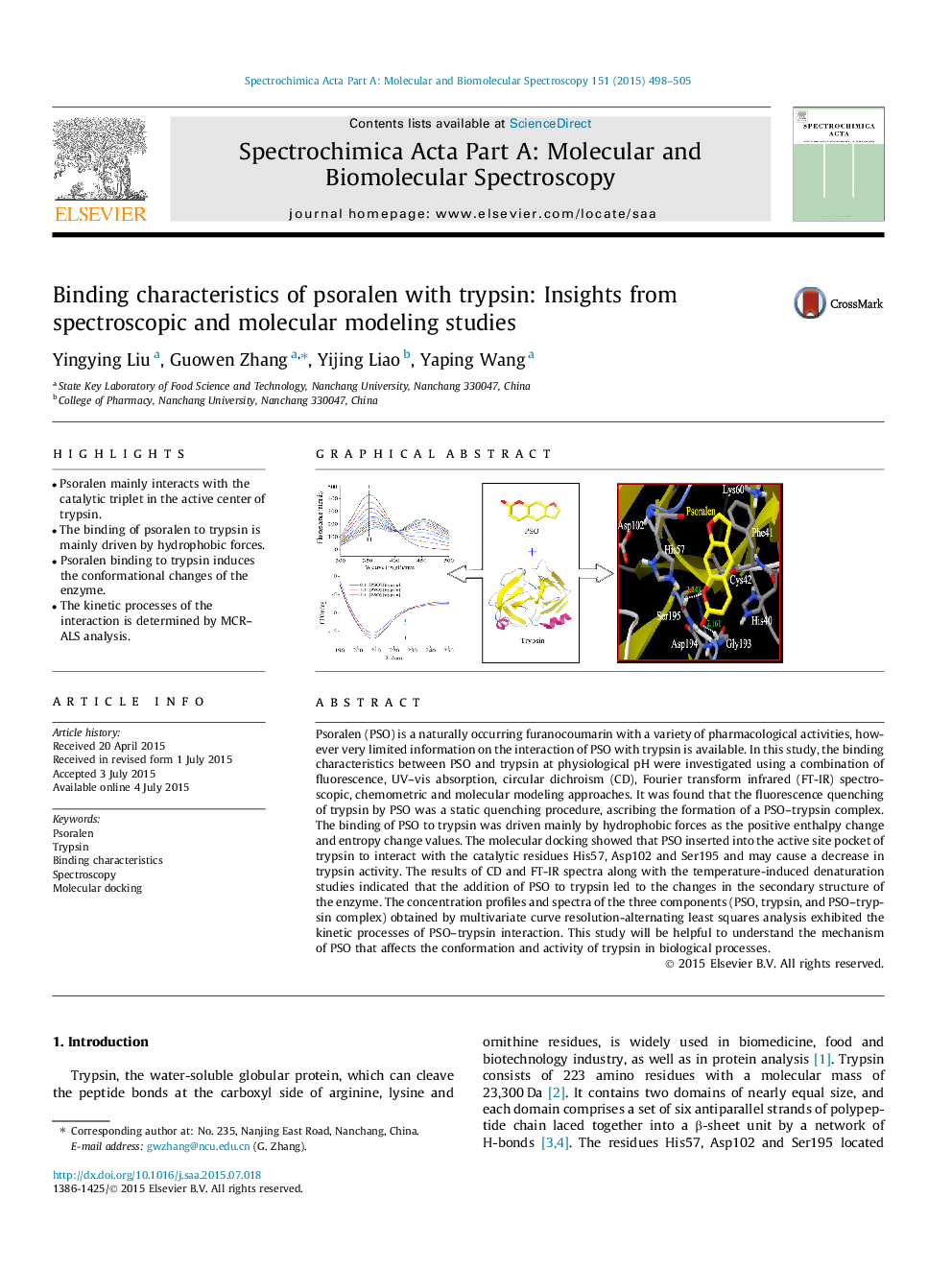
•Psoralen mainly interacts with the catalytic triplet in the active center of trypsin.•The binding of psoralen to trypsin is mainly driven by hydrophobic forces.•Psoralen binding to trypsin induces the conformational changes of the enzyme.•The kinetic processes of the interaction is determined by MCR–ALS analysis.
Psoralen (PSO) is a naturally occurring furanocoumarin with a variety of pharmacological activities, however very limited information on the interaction of PSO with trypsin is available. In this study, the binding characteristics between PSO and trypsin at physiological pH were investigated using a combination of fluorescence, UV–vis absorption, circular dichroism (CD), Fourier transform infrared (FT-IR) spectroscopic, chemometric and molecular modeling approaches. It was found that the fluorescence quenching of trypsin by PSO was a static quenching procedure, ascribing the formation of a PSO–trypsin complex. The binding of PSO to trypsin was driven mainly by hydrophobic forces as the positive enthalpy change and entropy change values. The molecular docking showed that PSO inserted into the active site pocket of trypsin to interact with the catalytic residues His57, Asp102 and Ser195 and may cause a decrease in trypsin activity. The results of CD and FT-IR spectra along with the temperature-induced denaturation studies indicated that the addition of PSO to trypsin led to the changes in the secondary structure of the enzyme. The concentration profiles and spectra of the three components (PSO, trypsin, and PSO–trypsin complex) obtained by multivariate curve resolution-alternating least squares analysis exhibited the kinetic processes of PSO–trypsin interaction. This study will be helpful to understand the mechanism of PSO that affects the conformation and activity of trypsin in biological processes.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide