| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230110 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 10 Pages |
•Designed and synthesized unique and versatile scaffolds.•Reactions within dual nature ionic liquids.•Activity against M. tuberculosis strains.
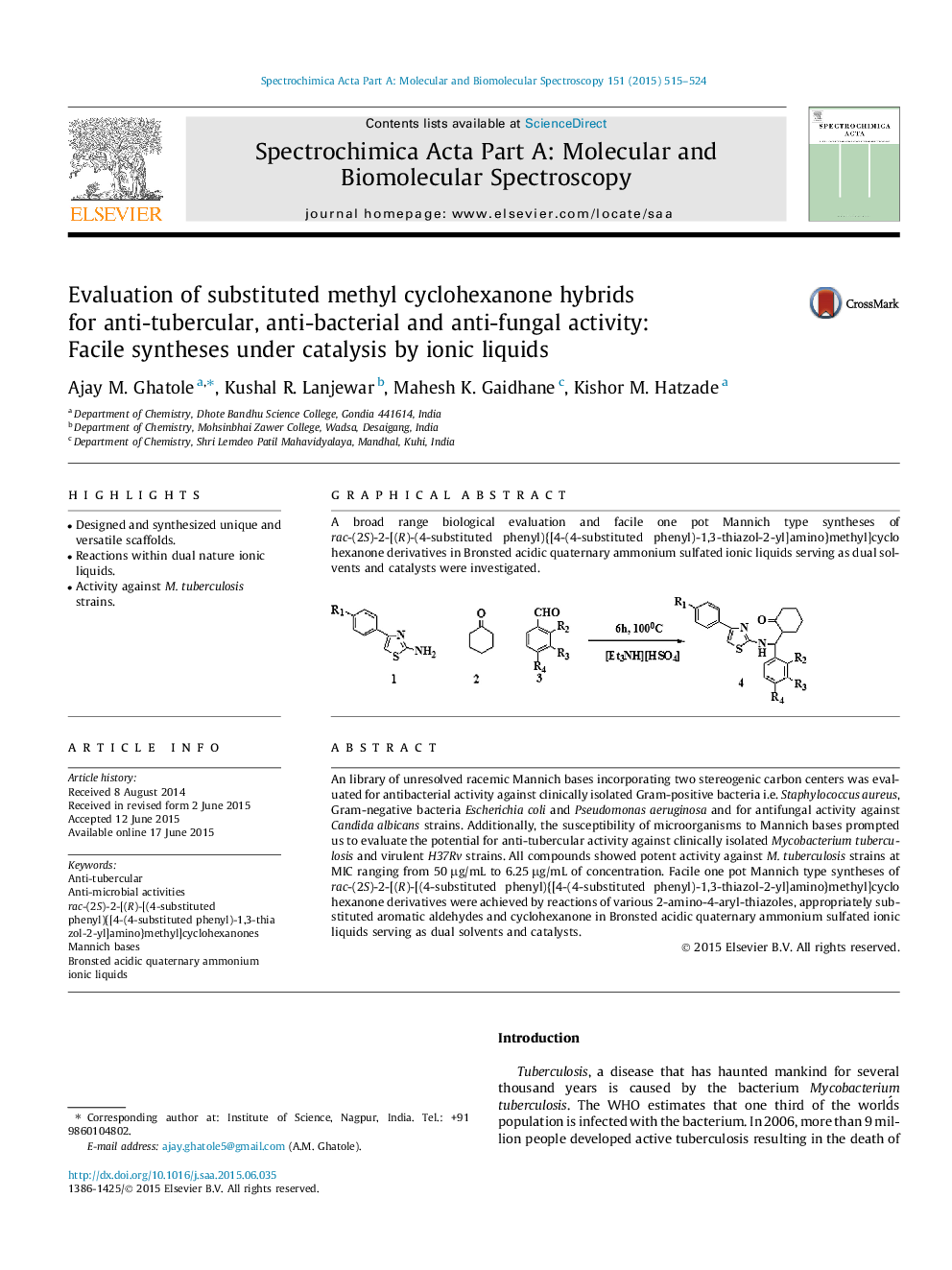
An library of unresolved racemic Mannich bases incorporating two stereogenic carbon centers was evaluated for antibacterial activity against clinically isolated Gram-positive bacteria i.e. Staphylococcus aureus, Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa and for antifungal activity against Candida albicans strains. Additionally, the susceptibility of microorganisms to Mannich bases prompted us to evaluate the potential for anti-tubercular activity against clinically isolated Mycobacterium tuberculosis and virulent H37Rv strains. All compounds showed potent activity against M. tuberculosis strains at MIC ranging from 50 μg/mL to 6.25 μg/mL of concentration. Facile one pot Mannich type syntheses of rac-(2S)-2-[(R)-[(4-substituted phenyl){[4-(4-substituted phenyl)-1,3-thiazol-2-yl]amino}methyl]cyclohexanone derivatives were achieved by reactions of various 2-amino-4-aryl-thiazoles, appropriately substituted aromatic aldehydes and cyclohexanone in Bronsted acidic quaternary ammonium sulfated ionic liquids serving as dual solvents and catalysts.
Graphical abstractA broad range biological evaluation and facile one pot Mannich type syntheses of rac-(2S)-2-[(R)-(4-substituted phenyl){[4-(4-substituted phenyl)-1,3-thiazol-2-yl]amino}methyl]cyclohexanone derivatives in Bronsted acidic quaternary ammonium sulfated ionic liquids serving as dual solvents and catalysts were investigated.Figure optionsDownload full-size imageDownload as PowerPoint slide