| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230119 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 7 Pages |
•The metal to ligand ratio was found to be 1:2 in all the complexes.•The Mössbauer spectra of the Fe(III) complex shows two doublets.•The newly synthesized complexes show better antimicrobial activity.•Co(II) complex is more viable at low concentrations against Human SkMEL cells.
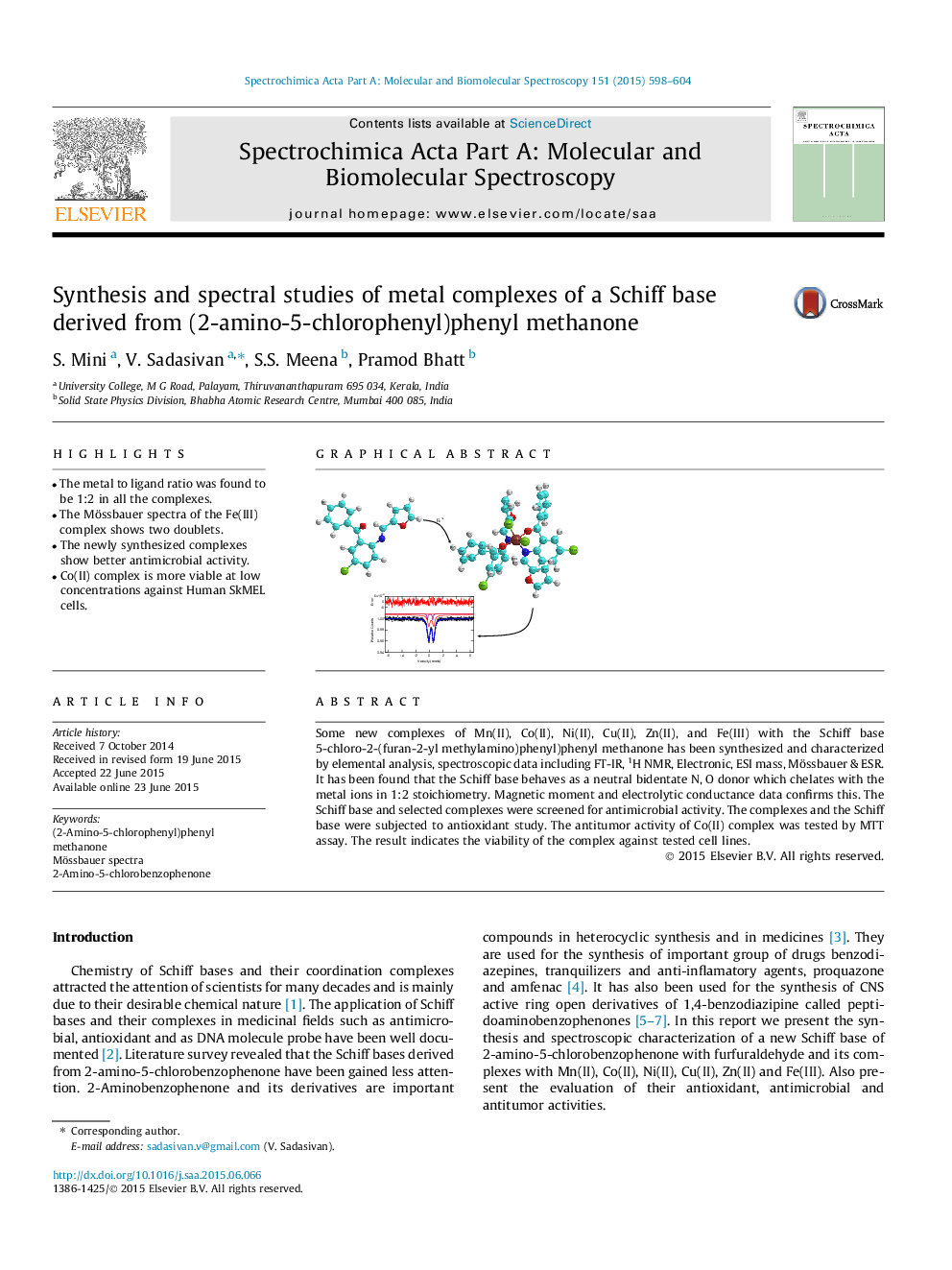
Some new complexes of Mn(II), Co(II), Ni(II), Cu(II), Zn(II), and Fe(III) with the Schiff base 5-chloro-2-(furan-2-yl methylamino)phenyl)phenyl methanone has been synthesized and characterized by elemental analysis, spectroscopic data including FT-IR, 1H NMR, Electronic, ESI mass, Mössbauer & ESR. It has been found that the Schiff base behaves as a neutral bidentate N, O donor which chelates with the metal ions in 1:2 stoichiometry. Magnetic moment and electrolytic conductance data confirms this. The Schiff base and selected complexes were screened for antimicrobial activity. The complexes and the Schiff base were subjected to antioxidant study. The antitumor activity of Co(II) complex was tested by MTT assay. The result indicates the viability of the complex against tested cell lines.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide