| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230121 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 5 Pages |
•We have studied the mineral rostite AlSO4(OH,F)·5(H2O).•Chemical analysis proves the presence of Al, F and S.•Multiple Raman and infrared bands in the OH stretching region are assigned to the stretching vibrations of water.•Raman and infrared bands are attributed to sulphate stretching and bending vibrations.•Vibrational spectroscopy enhances our knowledge of the molecular structure of rostite.
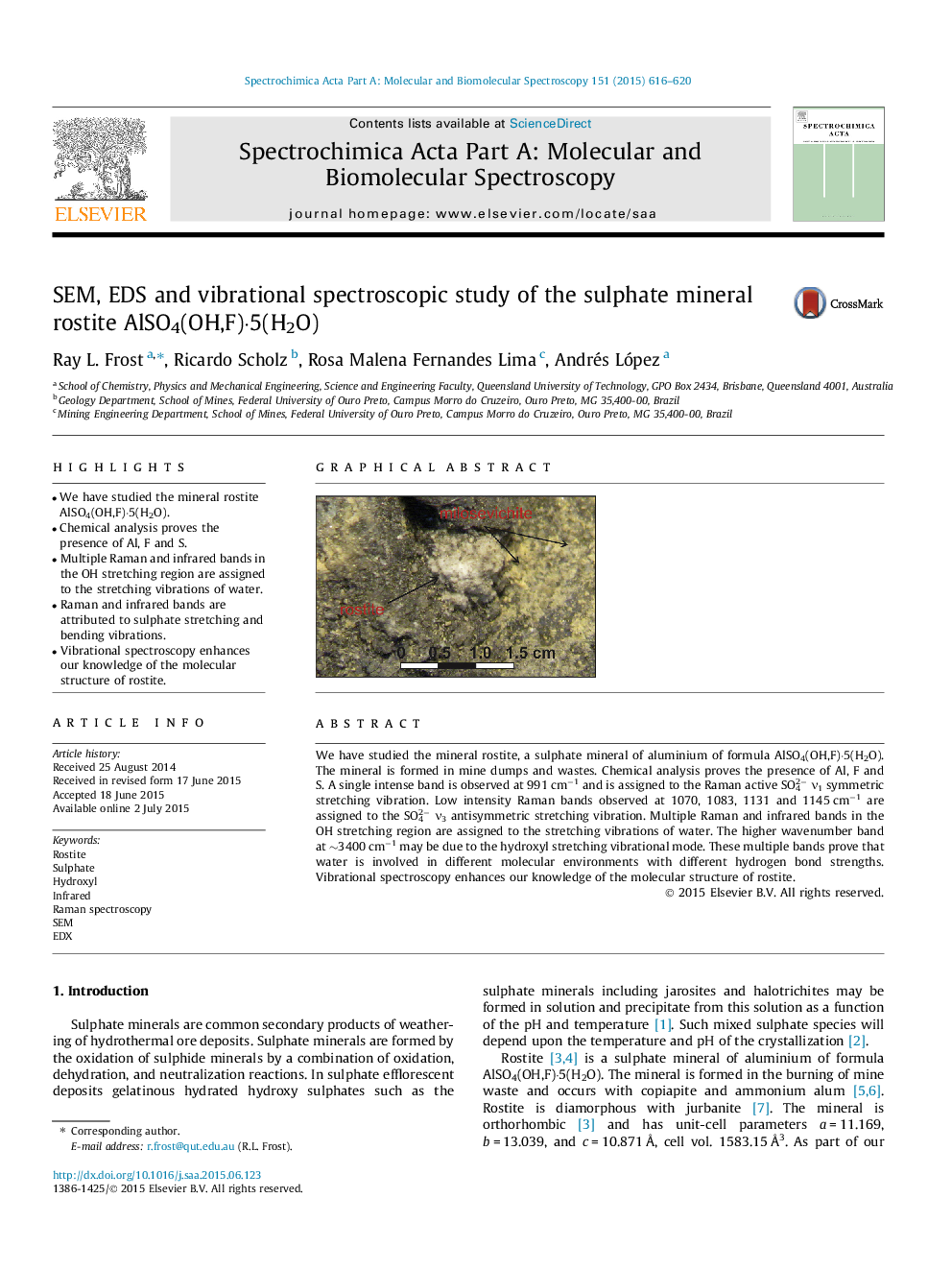
We have studied the mineral rostite, a sulphate mineral of aluminium of formula AlSO4(OH,F)·5(H2O). The mineral is formed in mine dumps and wastes. Chemical analysis proves the presence of Al, F and S. A single intense band is observed at 991 cm−1 and is assigned to the Raman active SO42− ν1 symmetric stretching vibration. Low intensity Raman bands observed at 1070, 1083, 1131 and 1145 cm−1 are assigned to the SO42− ν3 antisymmetric stretching vibration. Multiple Raman and infrared bands in the OH stretching region are assigned to the stretching vibrations of water. The higher wavenumber band at ∼3400 cm−1 may be due to the hydroxyl stretching vibrational mode. These multiple bands prove that water is involved in different molecular environments with different hydrogen bond strengths. Vibrational spectroscopy enhances our knowledge of the molecular structure of rostite.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide