| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230127 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 6 Pages |
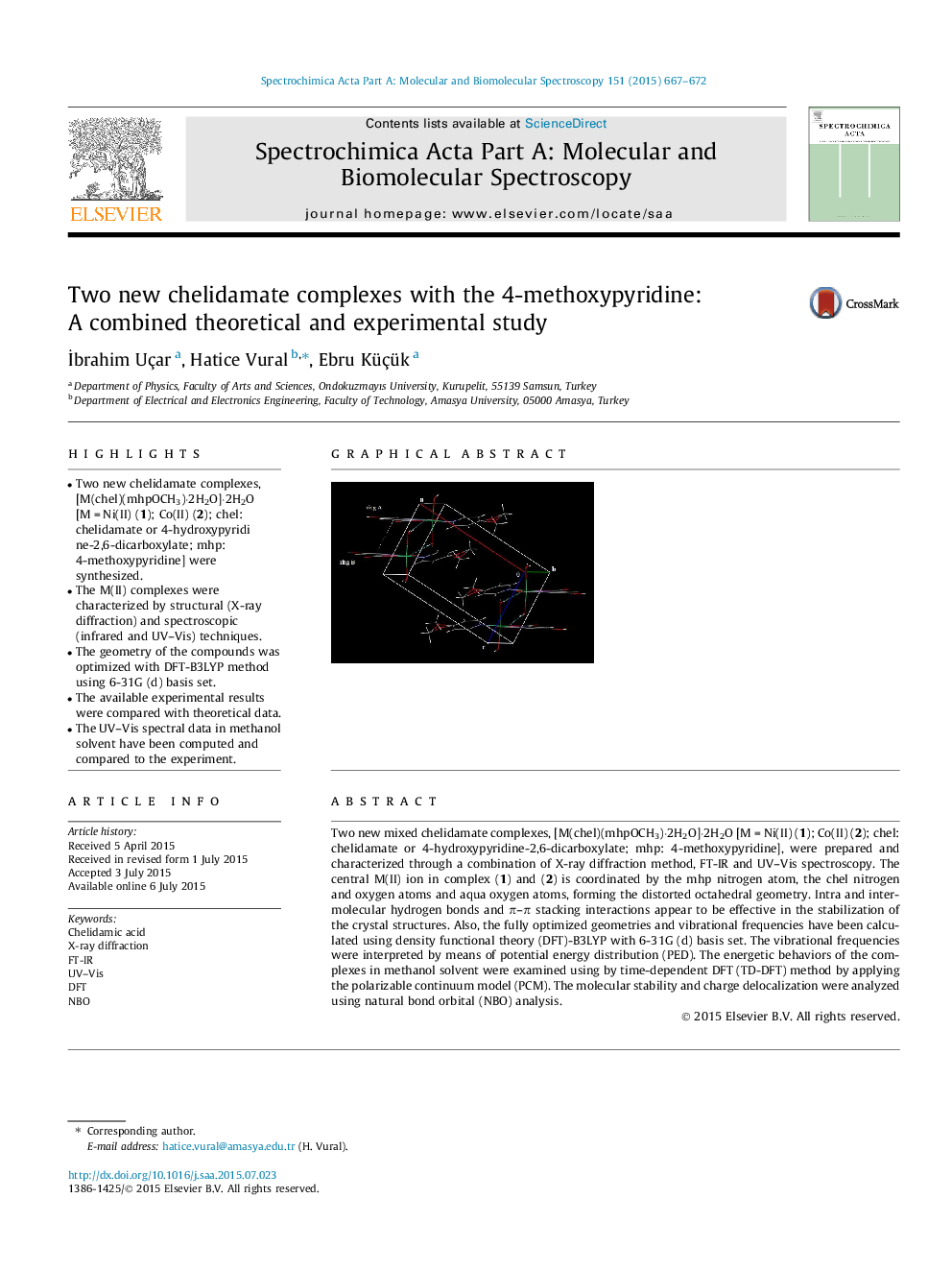
•Two new chelidamate complexes, [M(chel)(mhpOCH3)·2H2O]·2H2O [M = Ni(II) (1); Co(II) (2); chel: chelidamate or 4-hydroxypyridine-2,6-dicarboxylate; mhp: 4-methoxypyridine] were synthesized.•The M(II) complexes were characterized by structural (X-ray diffraction) and spectroscopic (infrared and UV–Vis) techniques.•The geometry of the compounds was optimized with DFT-B3LYP method using 6-31G (d) basis set.•The available experimental results were compared with theoretical data.•The UV–Vis spectral data in methanol solvent have been computed and compared to the experiment.
Two new mixed chelidamate complexes, [M(chel)(mhpOCH3)·2H2O]·2H2O [M = Ni(II) (1); Co(II) (2); chel: chelidamate or 4-hydroxypyridine-2,6-dicarboxylate; mhp: 4-methoxypyridine], were prepared and characterized through a combination of X-ray diffraction method, FT-IR and UV–Vis spectroscopy. The central M(II) ion in complex (1) and (2) is coordinated by the mhp nitrogen atom, the chel nitrogen and oxygen atoms and aqua oxygen atoms, forming the distorted octahedral geometry. Intra and intermolecular hydrogen bonds and π–π stacking interactions appear to be effective in the stabilization of the crystal structures. Also, the fully optimized geometries and vibrational frequencies have been calculated using density functional theory (DFT)-B3LYP with 6-31G (d) basis set. The vibrational frequencies were interpreted by means of potential energy distribution (PED). The energetic behaviors of the complexes in methanol solvent were examined using by time-dependent DFT (TD-DFT) method by applying the polarizable continuum model (PCM). The molecular stability and charge delocalization were analyzed using natural bond orbital (NBO) analysis.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide