| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230138 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 14 Pages |
Highlight•Red and blue shift HB and XB interactions, formyl halides–hypohalous acids intermolecular complexes.
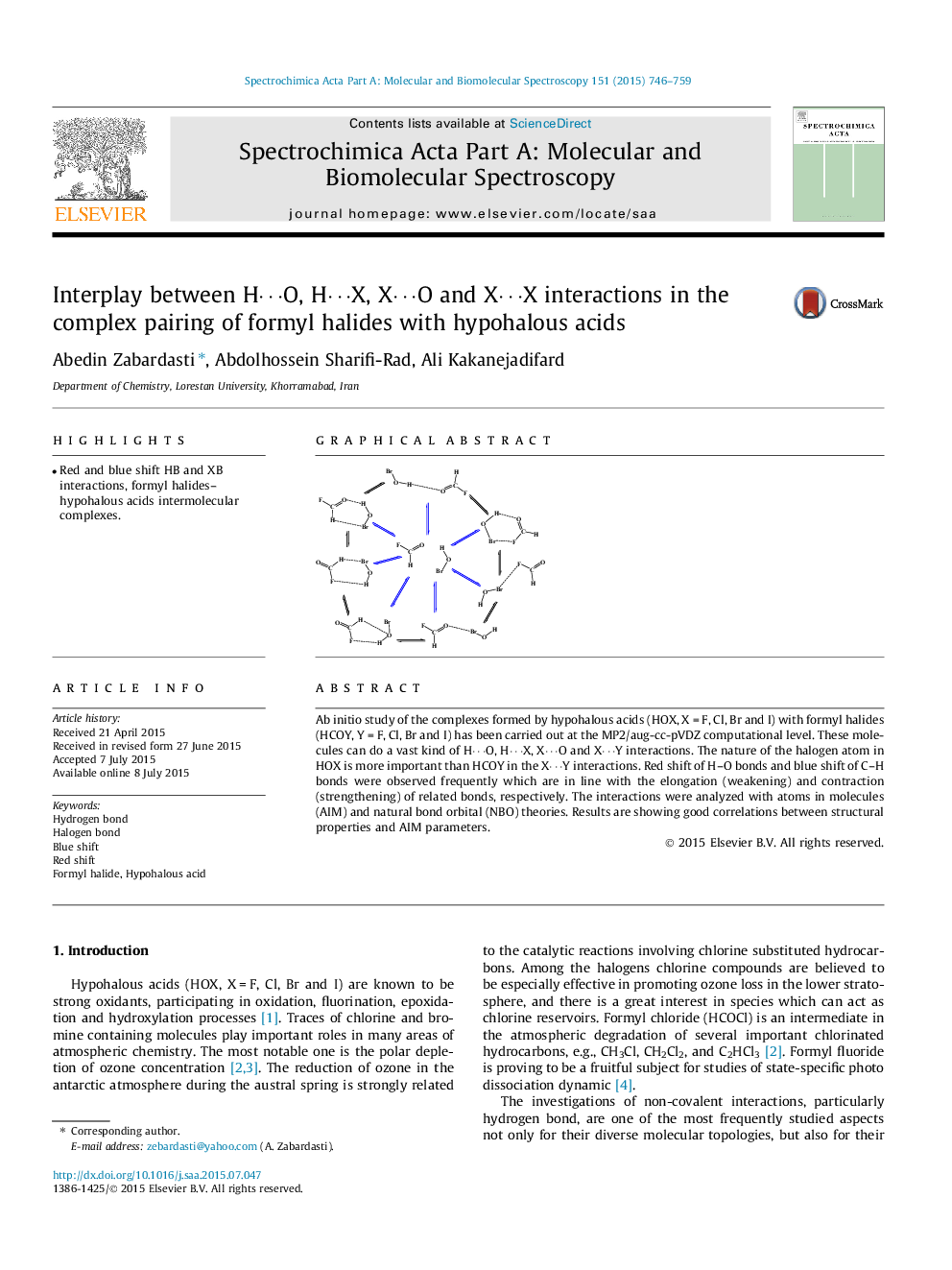
Ab initio study of the complexes formed by hypohalous acids (HOX, X = F, Cl, Br and I) with formyl halides (HCOY, Y = F, Cl, Br and I) has been carried out at the MP2/aug-cc-pVDZ computational level. These molecules can do a vast kind of H⋯O, H⋯X, X⋯O and X⋯Y interactions. The nature of the halogen atom in HOX is more important than HCOY in the X⋯Y interactions. Red shift of H–O bonds and blue shift of C–H bonds were observed frequently which are in line with the elongation (weakening) and contraction (strengthening) of related bonds, respectively. The interactions were analyzed with atoms in molecules (AIM) and natural bond orbital (NBO) theories. Results are showing good correlations between structural properties and AIM parameters.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide