| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230153 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
•Structural effect of Schiff bases on the stability constant of iodine complexes.•Spectrophotometric evaluation of ionization potentials of selected ligands.•Thermodynamic parameters well described by the modified Benesi–Hildebrand equation.
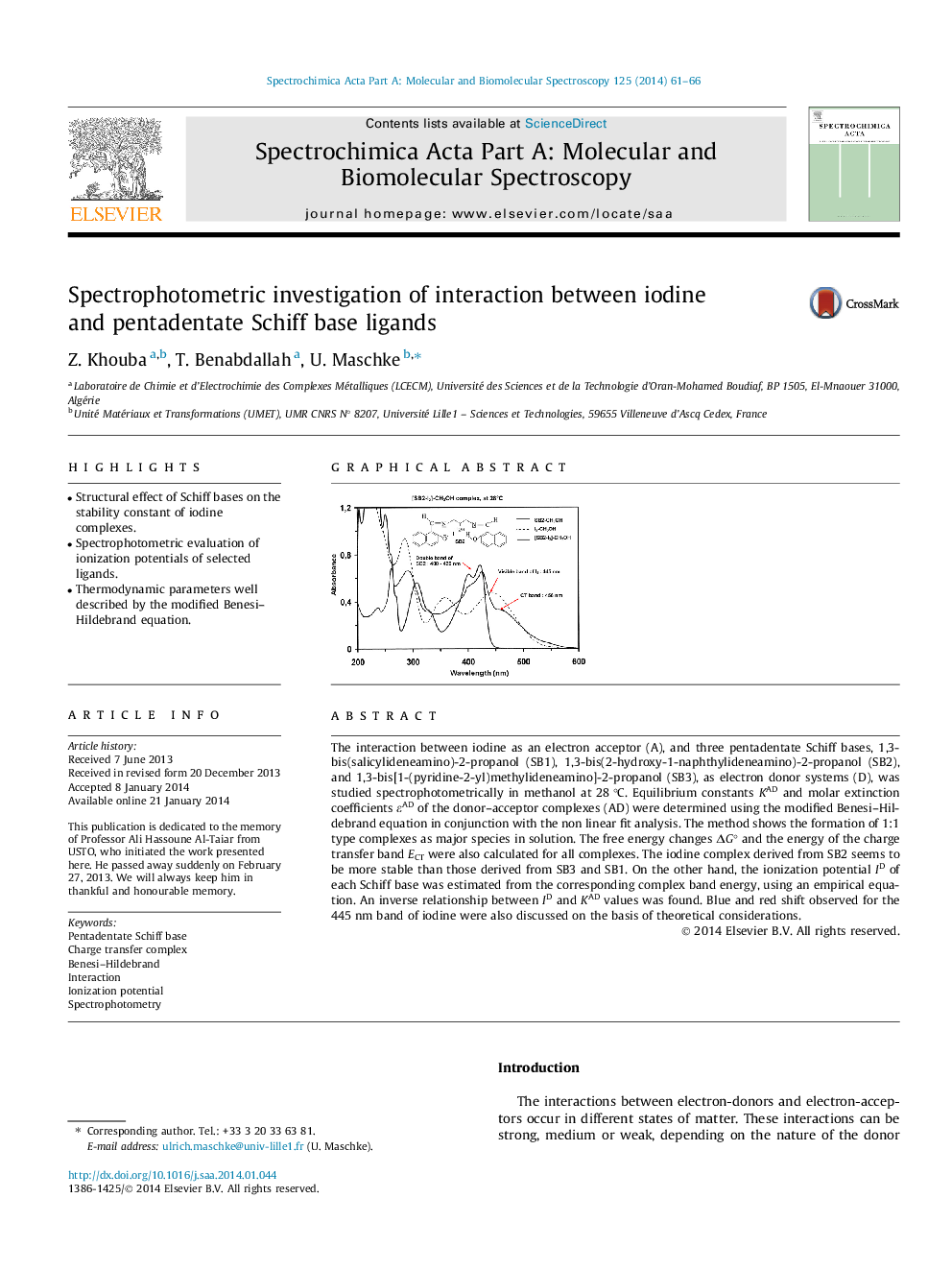
The interaction between iodine as an electron acceptor (A), and three pentadentate Schiff bases, 1,3-bis(salicylideneamino)-2-propanol (SB1), 1,3-bis(2-hydroxy-1-naphthylideneamino)-2-propanol (SB2), and 1,3-bis[1-(pyridine-2-yl)methylideneamino]-2-propanol (SB3), as electron donor systems (D), was studied spectrophotometrically in methanol at 28 °C. Equilibrium constants KAD and molar extinction coefficients εAD of the donor–acceptor complexes (AD) were determined using the modified Benesi–Hildebrand equation in conjunction with the non linear fit analysis. The method shows the formation of 1:1 type complexes as major species in solution. The free energy changes ΔG° and the energy of the charge transfer band ECT were also calculated for all complexes. The iodine complex derived from SB2 seems to be more stable than those derived from SB3 and SB1. On the other hand, the ionization potential ID of each Schiff base was estimated from the corresponding complex band energy, using an empirical equation. An inverse relationship between ID and KAD values was found. Blue and red shift observed for the 445 nm band of iodine were also discussed on the basis of theoretical considerations.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide