| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230157 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 9 Pages |
•Ground state complexation on fullerene–monoporphyrin (1) complexes in solution.•Fluorescence study elicits efficient quenching of 1 in presence of C60 and C70.•Time-resolved emission study reveals static quenching mechanism.•Ab initio calculations explore geometric alignment of fullerenes towards 1 in vacuo.
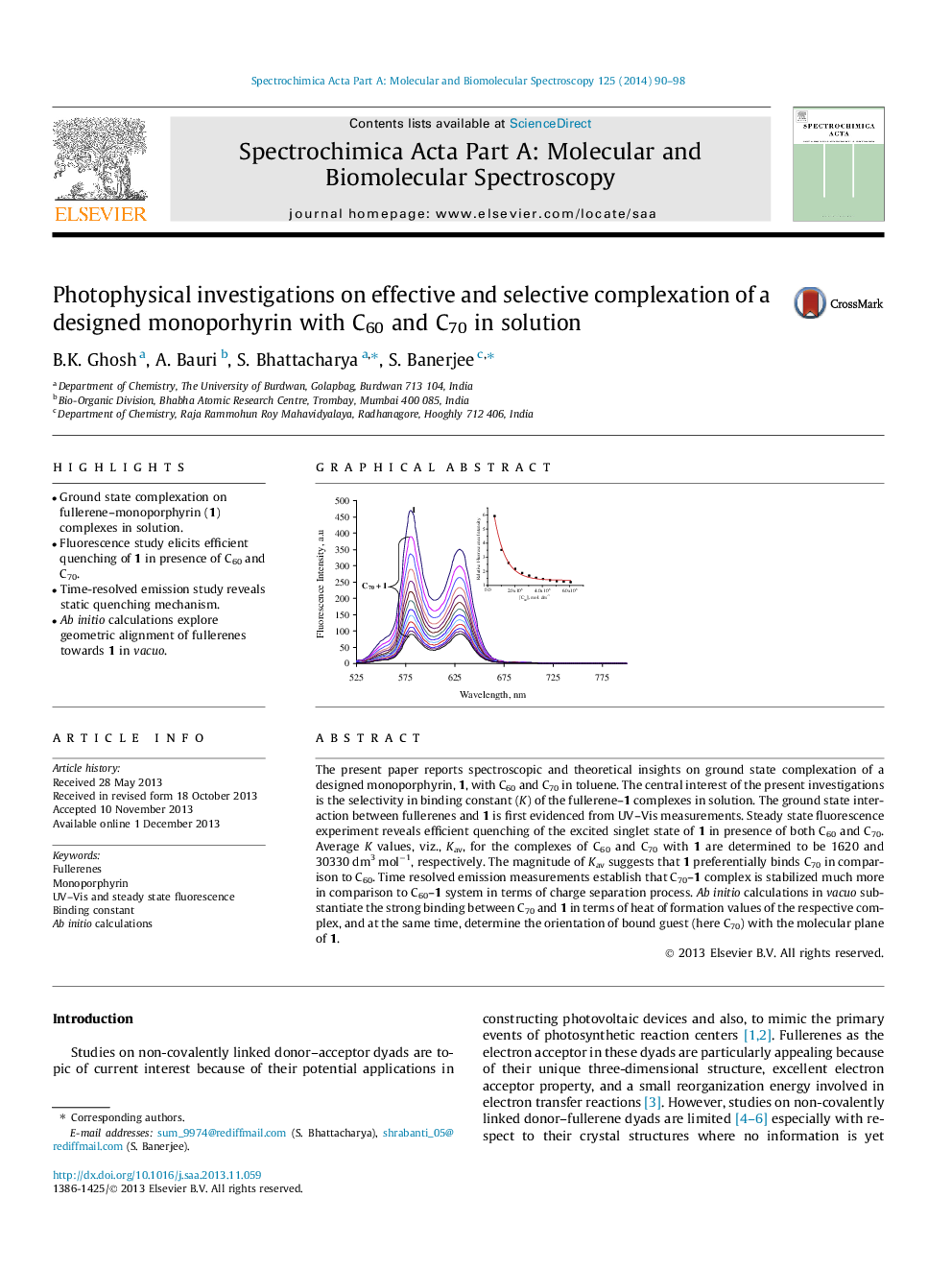
The present paper reports spectroscopic and theoretical insights on ground state complexation of a designed monoporphyrin, 1, with C60 and C70 in toluene. The central interest of the present investigations is the selectivity in binding constant (K) of the fullerene–1 complexes in solution. The ground state interaction between fullerenes and 1 is first evidenced from UV–Vis measurements. Steady state fluorescence experiment reveals efficient quenching of the excited singlet state of 1 in presence of both C60 and C70. Average K values, viz., Kav, for the complexes of C60 and C70 with 1 are determined to be 1620 and 30330 dm3 mol−1, respectively. The magnitude of Kav suggests that 1 preferentially binds C70 in comparison to C60. Time resolved emission measurements establish that C70–1 complex is stabilized much more in comparison to C60–1 system in terms of charge separation process. Ab initio calculations in vacuo substantiate the strong binding between C70 and 1 in terms of heat of formation values of the respective complex, and at the same time, determine the orientation of bound guest (here C70) with the molecular plane of 1.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide