| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230189 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 8 Pages |
•New azo-azomethine dyes based on 1,2,4-triazole have been synthesized.•TD-DFT and NBO studies on azo-azomethine dyes have been reported.•1H NMR chemical shifts of the dyes studied by GIAO/DFT and CSGT/DFT methods.
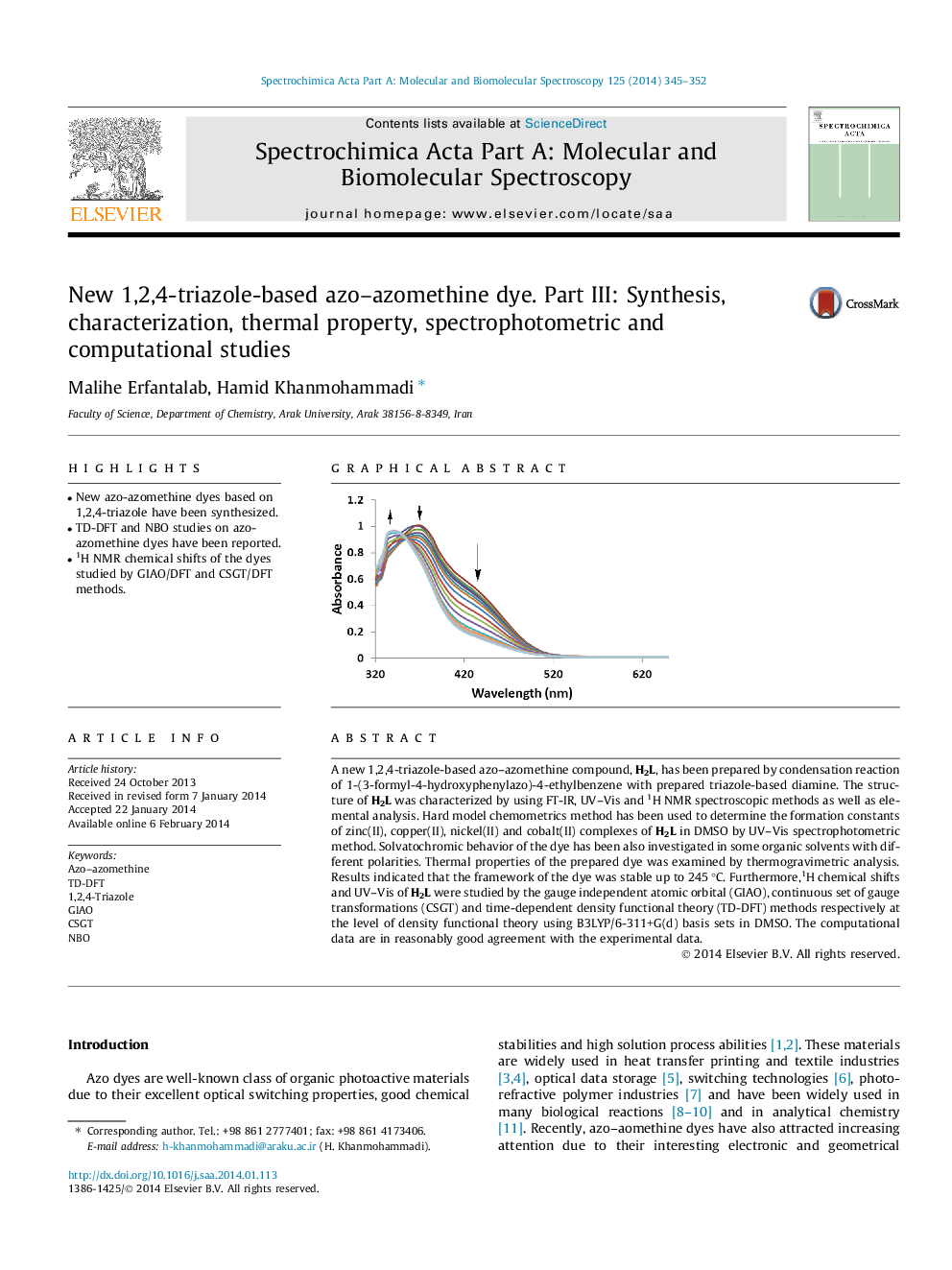
A new 1,2,4-triazole-based azo–azomethine compound, H2L, has been prepared by condensation reaction of 1-(3-formyl-4-hydroxyphenylazo)-4-ethylbenzene with prepared triazole-based diamine. The structure of H2L was characterized by using FT-IR, UV–Vis and 1H NMR spectroscopic methods as well as elemental analysis. Hard model chemometrics method has been used to determine the formation constants of zinc(II), copper(II), nickel(II) and cobalt(II) complexes of H2L in DMSO by UV–Vis spectrophotometric method. Solvatochromic behavior of the dye has been also investigated in some organic solvents with different polarities. Thermal properties of the prepared dye was examined by thermogravimetric analysis. Results indicated that the framework of the dye was stable up to 245 °C. Furthermore,1H chemical shifts and UV–Vis of H2L were studied by the gauge independent atomic orbital (GIAO), continuous set of gauge transformations (CSGT) and time-dependent density functional theory (TD-DFT) methods respectively at the level of density functional theory using B3LYP/6-311+G(d) basis sets in DMSO. The computational data are in reasonably good agreement with the experimental data.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide