| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230194 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 7 Pages |
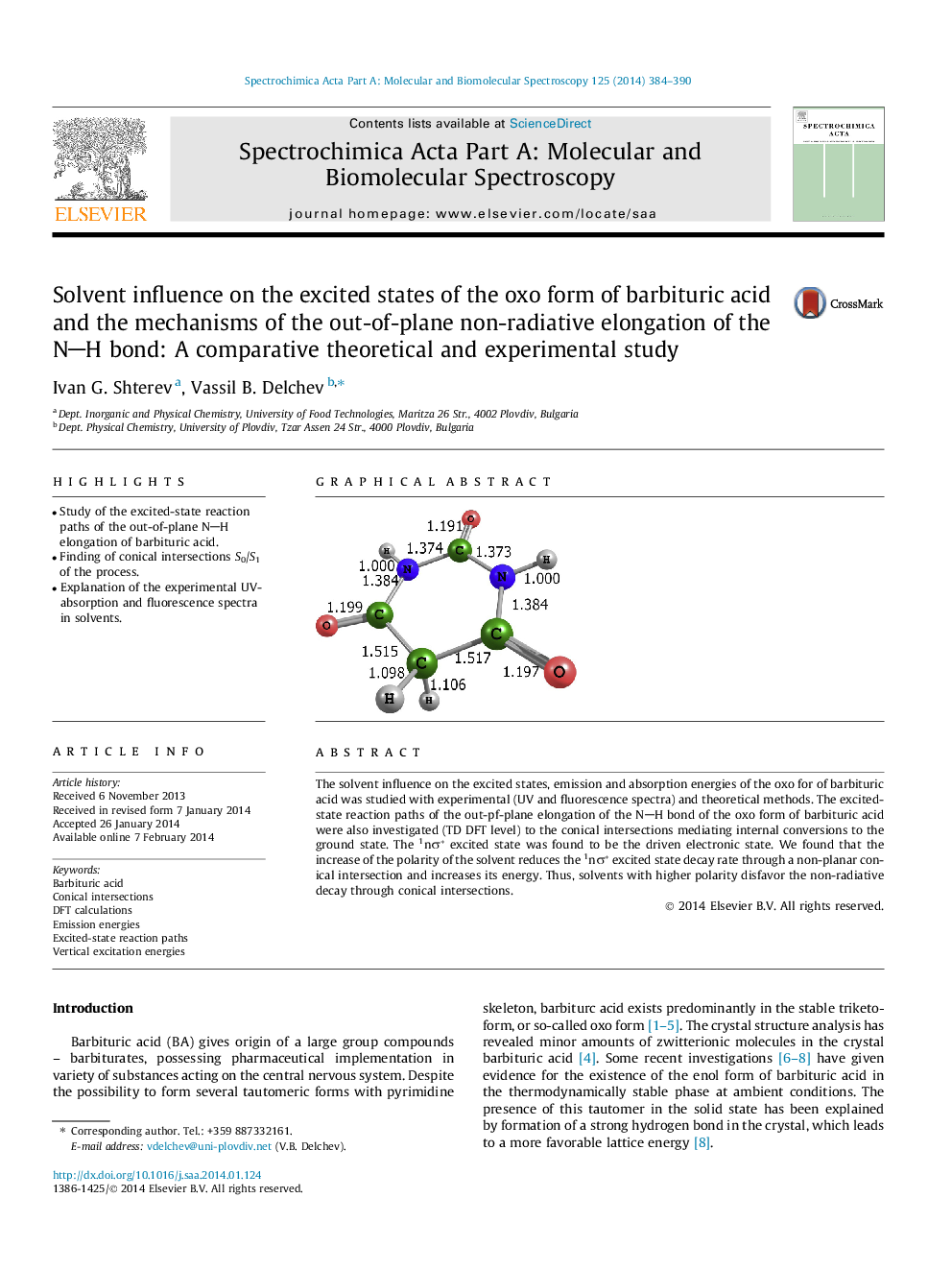
•Study of the excited-state reaction paths of the out-of-plane NH elongation of barbituric acid.•Finding of conical intersections S0/S1 of the process.•Explanation of the experimental UV-absorption and fluorescence spectra in solvents.
The solvent influence on the excited states, emission and absorption energies of the oxo for of barbituric acid was studied with experimental (UV and fluorescence spectra) and theoretical methods. The excited-state reaction paths of the out-pf-plane elongation of the NH bond of the oxo form of barbituric acid were also investigated (TD DFT level) to the conical intersections mediating internal conversions to the ground state. The 1nσ* excited state was found to be the driven electronic state. We found that the increase of the polarity of the solvent reduces the 1nσ* excited state decay rate through a non-planar conical intersection and increases its energy. Thus, solvents with higher polarity disfavor the non-radiative decay through conical intersections.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide