| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230195 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 5 Pages |
•The fluorescence quenching of bovine serum albumin (BSA) by pyrene is static.•The pyrene–BSA complex has been formed.•The energy transfer of BSA to pyrene occurs.•Van der Waals forces and hydrogen bonds play major roles in the binding process.•The conformational changes indicate that pyrene induces damage to BSA.
This paper was designed to investigate the interaction of pyrene with bovine serum albumin (BSA) under physiological condition by spectroscopic methods. Spectroscopic analysis of the emission quenching revealed that the quenching mechanism of BSA by pyrene was static. The binding sites and constants of pyrene–BSA complex were observed to be 1.20 and 2.63 × 106 L mol−1 at 298 K, respectively. The enthalpy change (ΔH) and entropy change (ΔS) revealed that van der Waals forces and hydrogen bonds stabilized the pyrene–BSA complex. Energy transfer from tryptophan to pyrene occurred by a FRET (fluorescence resonance energy transfer) mechanism, and the distance (r = 2.72 nm) had been determined. The results of synchronous, three-dimensional fluorescence, and circular dichroism spectra showed that the pyrene induced conformational changes of BSA.
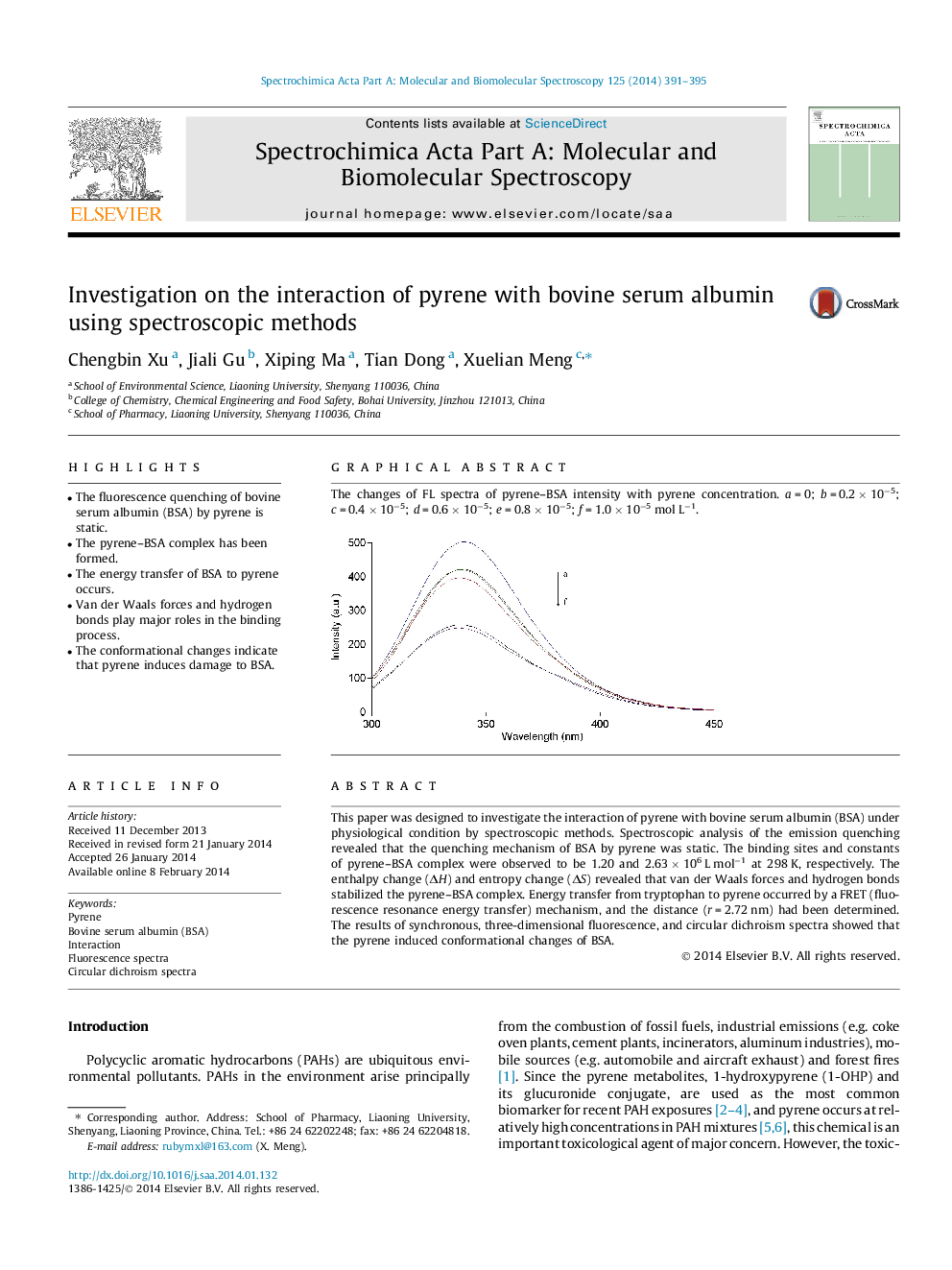
Graphical abstractThe changes of FL spectra of pyrene–BSA intensity with pyrene concentration. a = 0; b = 0.2 × 10−5; c = 0.4 × 10−5; d = 0.6 × 10−5; e = 0.8 × 10−5; f = 1.0 × 10−5 mol L−1.Figure optionsDownload full-size imageDownload as PowerPoint slide