| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230202 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 4 Pages |
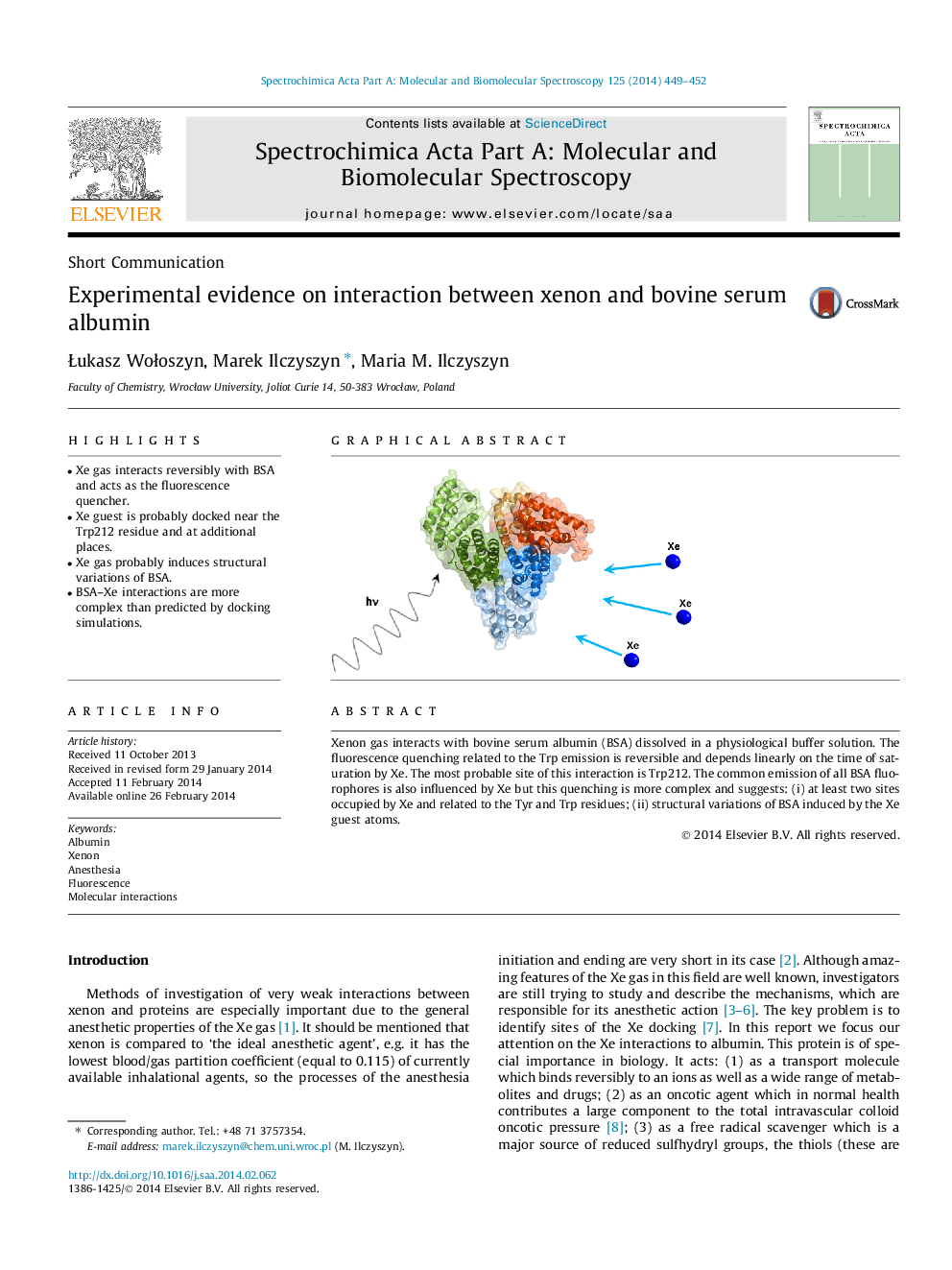
•Xe gas interacts reversibly with BSA and acts as the fluorescence quencher.•Xe guest is probably docked near the Trp212 residue and at additional places.•Xe gas probably induces structural variations of BSA.•BSA–Xe interactions are more complex than predicted by docking simulations.
Xenon gas interacts with bovine serum albumin (BSA) dissolved in a physiological buffer solution. The fluorescence quenching related to the Trp emission is reversible and depends linearly on the time of saturation by Xe. The most probable site of this interaction is Trp212. The common emission of all BSA fluorophores is also influenced by Xe but this quenching is more complex and suggests: (i) at least two sites occupied by Xe and related to the Tyr and Trp residues; (ii) structural variations of BSA induced by the Xe guest atoms.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide