| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230275 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 7 Pages |
•The use of water-soluble Mn-doped ZnS QDs as chemiluminescence emitter.•The use of ionic liquid/copper complex as an efficient and green catalyst.•The determination of folic acid in commercial drugs with low detection limit.•The common ions cannot interfere to the detection of folic acid.
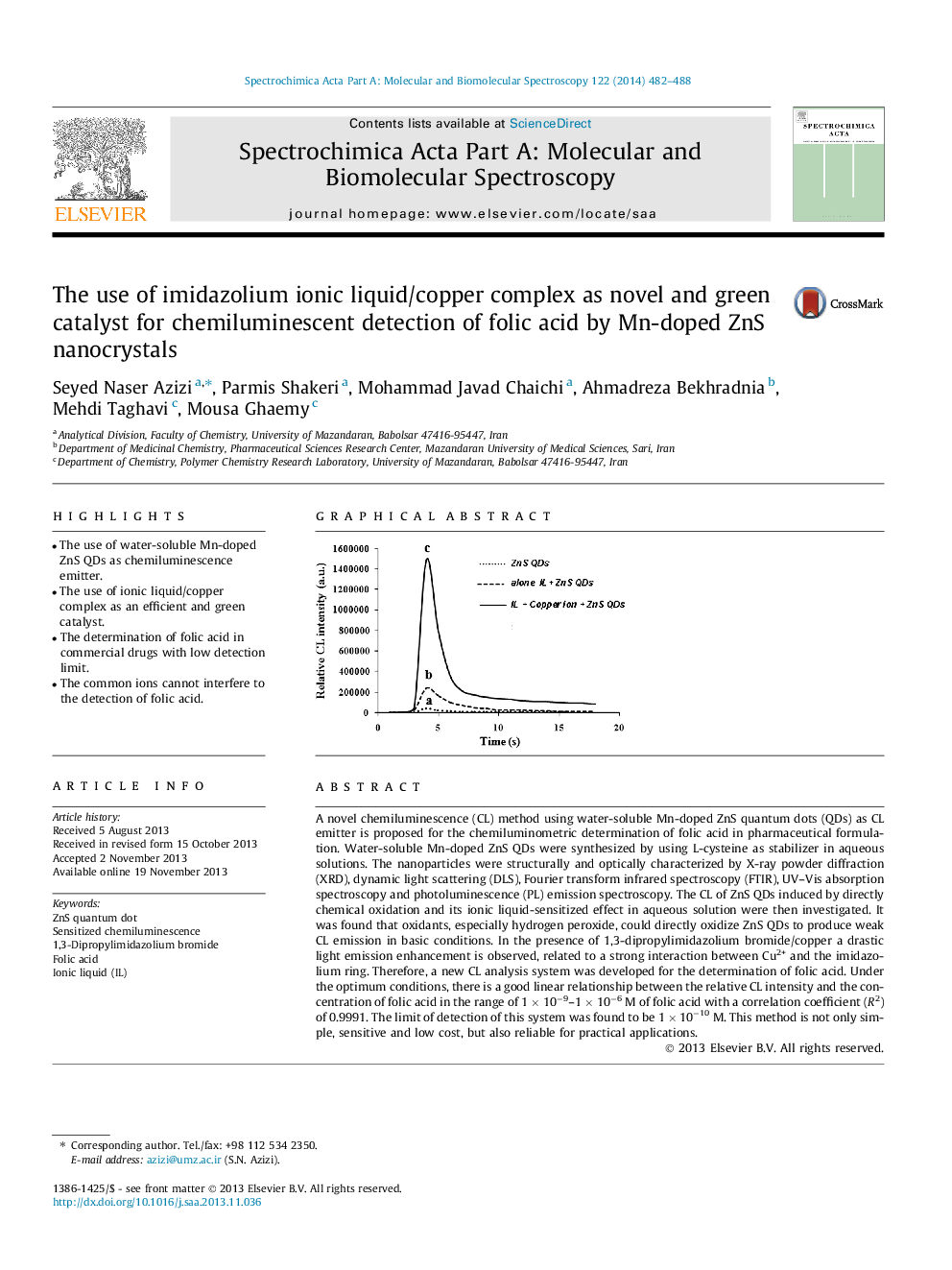
A novel chemiluminescence (CL) method using water-soluble Mn-doped ZnS quantum dots (QDs) as CL emitter is proposed for the chemiluminometric determination of folic acid in pharmaceutical formulation. Water-soluble Mn-doped ZnS QDs were synthesized by using L-cysteine as stabilizer in aqueous solutions. The nanoparticles were structurally and optically characterized by X-ray powder diffraction (XRD), dynamic light scattering (DLS), Fourier transform infrared spectroscopy (FTIR), UV–Vis absorption spectroscopy and photoluminescence (PL) emission spectroscopy. The CL of ZnS QDs induced by directly chemical oxidation and its ionic liquid-sensitized effect in aqueous solution were then investigated. It was found that oxidants, especially hydrogen peroxide, could directly oxidize ZnS QDs to produce weak CL emission in basic conditions. In the presence of 1,3-dipropylimidazolium bromide/copper a drastic light emission enhancement is observed, related to a strong interaction between Cu2+ and the imidazolium ring. Therefore, a new CL analysis system was developed for the determination of folic acid. Under the optimum conditions, there is a good linear relationship between the relative CL intensity and the concentration of folic acid in the range of 1 × 10−9–1 × 10−6 M of folic acid with a correlation coefficient (R2) of 0.9991. The limit of detection of this system was found to be 1 × 10−10 M. This method is not only simple, sensitive and low cost, but also reliable for practical applications.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide