| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230290 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 8 Pages |
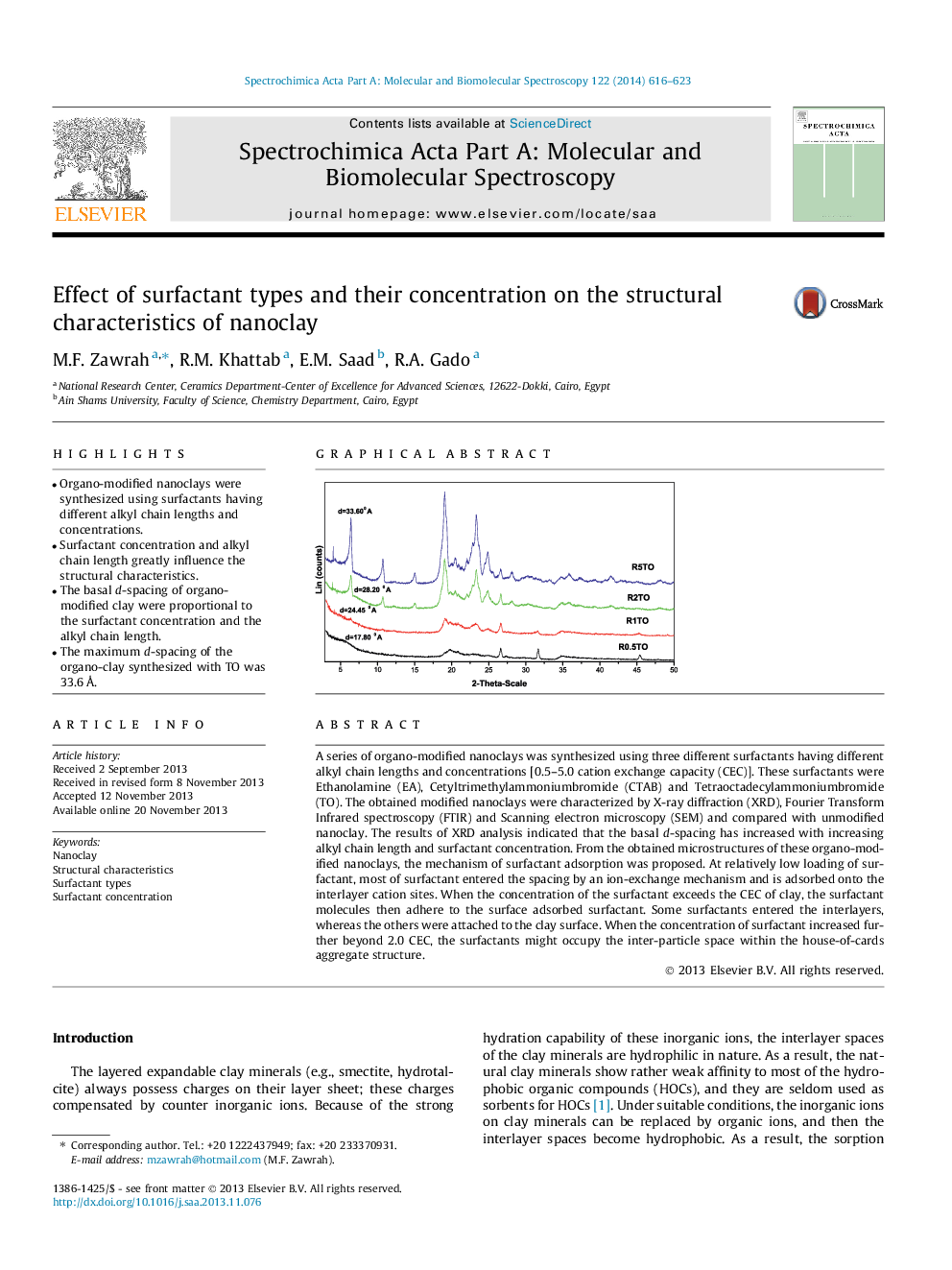
•Organo-modified nanoclays were synthesized using surfactants having different alkyl chain lengths and concentrations.•Surfactant concentration and alkyl chain length greatly influence the structural characteristics.•The basal d-spacing of organo-modified clay were proportional to the surfactant concentration and the alkyl chain length.•The maximum d-spacing of the organo-clay synthesized with TO was 33.6 Å.
A series of organo-modified nanoclays was synthesized using three different surfactants having different alkyl chain lengths and concentrations [0.5–5.0 cation exchange capacity (CEC)]. These surfactants were Ethanolamine (EA), Cetyltrimethylammoniumbromide (CTAB) and Tetraoctadecylammoniumbromide (TO). The obtained modified nanoclays were characterized by X-ray diffraction (XRD), Fourier Transform Infrared spectroscopy (FTIR) and Scanning electron microscopy (SEM) and compared with unmodified nanoclay. The results of XRD analysis indicated that the basal d-spacing has increased with increasing alkyl chain length and surfactant concentration. From the obtained microstructures of these organo-modified nanoclays, the mechanism of surfactant adsorption was proposed. At relatively low loading of surfactant, most of surfactant entered the spacing by an ion-exchange mechanism and is adsorbed onto the interlayer cation sites. When the concentration of the surfactant exceeds the CEC of clay, the surfactant molecules then adhere to the surface adsorbed surfactant. Some surfactants entered the interlayers, whereas the others were attached to the clay surface. When the concentration of surfactant increased further beyond 2.0 CEC, the surfactants might occupy the inter-particle space within the house-of-cards aggregate structure.
Graphical abstractXRD diffraction patterns of organo-modified nanoclays by TO. The results of XRD analysis indicated that the basal d-spacing has increased with increasing alkyl chain length and surfactant concentration.Figure optionsDownload full-size imageDownload as PowerPoint slide