| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230332 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 11 Pages |
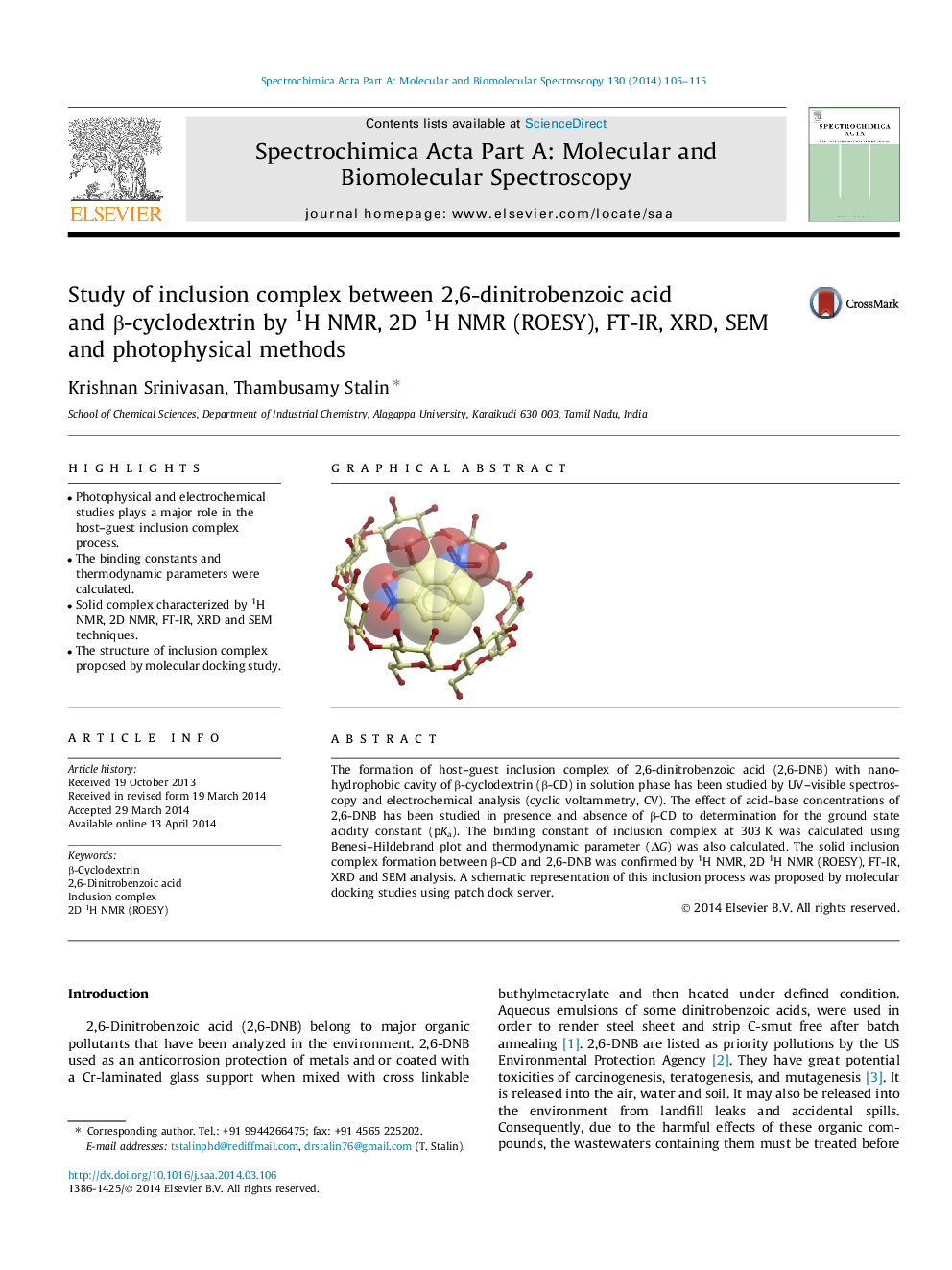
•Photophysical and electrochemical studies plays a major role in the host–guest inclusion complex process.•The binding constants and thermodynamic parameters were calculated.•Solid complex characterized by 1H NMR, 2D NMR, FT-IR, XRD and SEM techniques.•The structure of inclusion complex proposed by molecular docking study.
The formation of host–guest inclusion complex of 2,6-dinitrobenzoic acid (2,6-DNB) with nano-hydrophobic cavity of β-cyclodextrin (β-CD) in solution phase has been studied by UV–visible spectroscopy and electrochemical analysis (cyclic voltammetry, CV). The effect of acid–base concentrations of 2,6-DNB has been studied in presence and absence of β-CD to determination for the ground state acidity constant (pKa). The binding constant of inclusion complex at 303 K was calculated using Benesi–Hildebrand plot and thermodynamic parameter (ΔG) was also calculated. The solid inclusion complex formation between β-CD and 2,6-DNB was confirmed by 1H NMR, 2D 1H NMR (ROESY), FT-IR, XRD and SEM analysis. A schematic representation of this inclusion process was proposed by molecular docking studies using patch dock server.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide