| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230337 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 9 Pages |
•The FT-IR and FT-Raman spectra of 1-methyl-2-phenyl benzimidazole were analyzed.•Charge transfer characteristics were examined by HOMO–LUMO and NBO analysis.•Calculations were carried out at B3LYP/6-311 + G(d,p) and 6-311++G(d,p) basis sets.•MEP surface provide information about donor and acceptor atoms in the molecule.•Thermodynamic properties were determined at different temperatures.
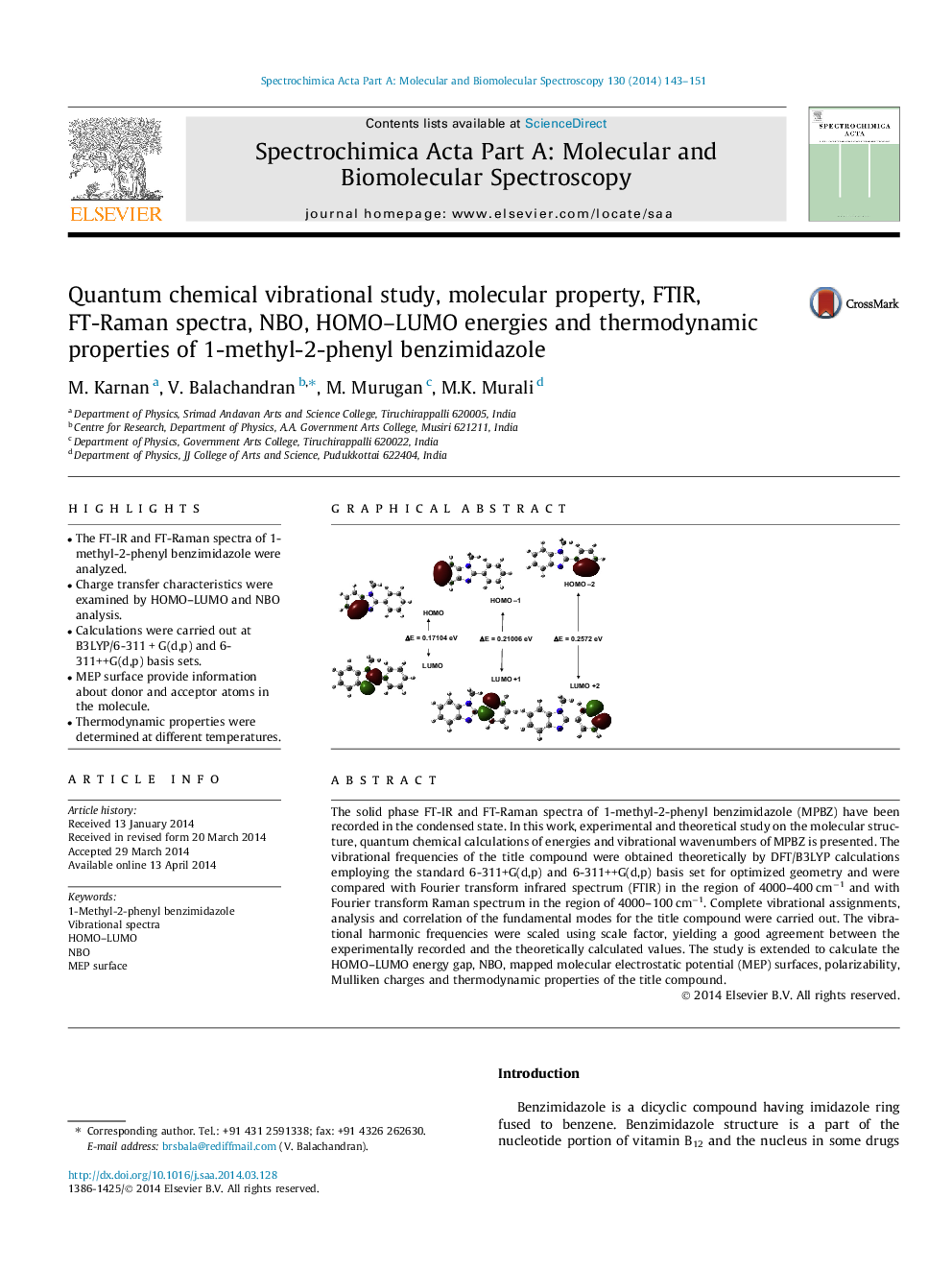
The solid phase FT-IR and FT-Raman spectra of 1-methyl-2-phenyl benzimidazole (MPBZ) have been recorded in the condensed state. In this work, experimental and theoretical study on the molecular structure, quantum chemical calculations of energies and vibrational wavenumbers of MPBZ is presented. The vibrational frequencies of the title compound were obtained theoretically by DFT/B3LYP calculations employing the standard 6-311+G(d,p) and 6-311++G(d,p) basis set for optimized geometry and were compared with Fourier transform infrared spectrum (FTIR) in the region of 4000–400 cm−1 and with Fourier transform Raman spectrum in the region of 4000–100 cm−1. Complete vibrational assignments, analysis and correlation of the fundamental modes for the title compound were carried out. The vibrational harmonic frequencies were scaled using scale factor, yielding a good agreement between the experimentally recorded and the theoretically calculated values. The study is extended to calculate the HOMO–LUMO energy gap, NBO, mapped molecular electrostatic potential (MEP) surfaces, polarizability, Mulliken charges and thermodynamic properties of the title compound.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide