| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230339 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 14 Pages |
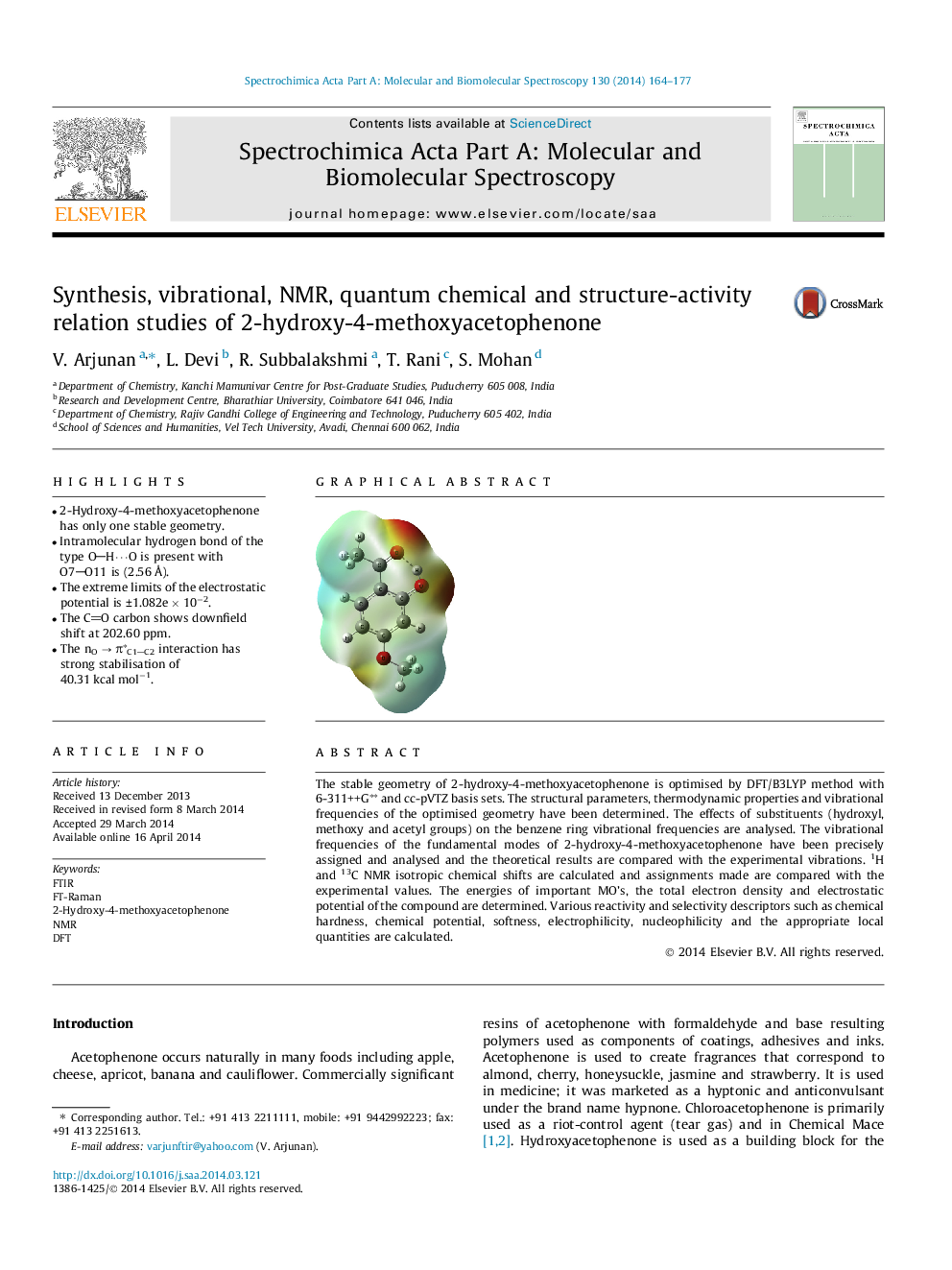
•2-Hydroxy-4-methoxyacetophenone has only one stable geometry.•Intramolecular hydrogen bond of the type OH⋯O is present with O7O11 is (2.56 Å).•The extreme limits of the electrostatic potential is ±1.082e × 10−2.•The CO carbon shows downfield shift at 202.60 ppm.•The nO → π∗C1C2 interaction has strong stabilisation of 40.31 kcal mol−1.
The stable geometry of 2-hydroxy-4-methoxyacetophenone is optimised by DFT/B3LYP method with 6-311++G∗∗ and cc-pVTZ basis sets. The structural parameters, thermodynamic properties and vibrational frequencies of the optimised geometry have been determined. The effects of substituents (hydroxyl, methoxy and acetyl groups) on the benzene ring vibrational frequencies are analysed. The vibrational frequencies of the fundamental modes of 2-hydroxy-4-methoxyacetophenone have been precisely assigned and analysed and the theoretical results are compared with the experimental vibrations. 1H and 13C NMR isotropic chemical shifts are calculated and assignments made are compared with the experimental values. The energies of important MO’s, the total electron density and electrostatic potential of the compound are determined. Various reactivity and selectivity descriptors such as chemical hardness, chemical potential, softness, electrophilicity, nucleophilicity and the appropriate local quantities are calculated.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide