| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230353 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 7 Pages |
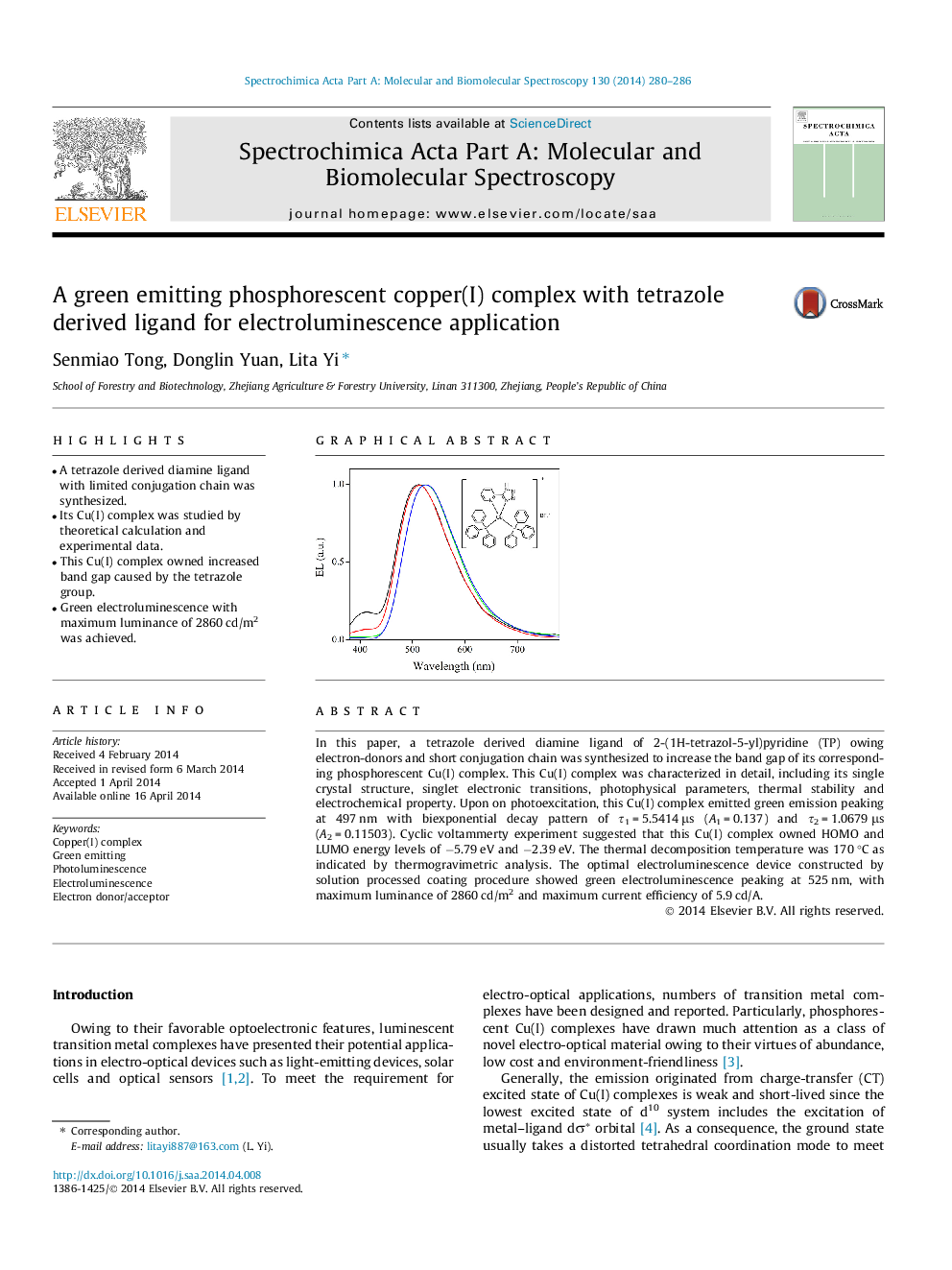
•A tetrazole derived diamine ligand with limited conjugation chain was synthesized.•Its Cu(I) complex was studied by theoretical calculation and experimental data.•This Cu(I) complex owned increased band gap caused by the tetrazole group.•Green electroluminescence with maximum luminance of 2860 cd/m2 was achieved.
In this paper, a tetrazole derived diamine ligand of 2-(1H-tetrazol-5-yl)pyridine (TP) owing electron-donors and short conjugation chain was synthesized to increase the band gap of its corresponding phosphorescent Cu(I) complex. This Cu(I) complex was characterized in detail, including its single crystal structure, singlet electronic transitions, photophysical parameters, thermal stability and electrochemical property. Upon on photoexcitation, this Cu(I) complex emitted green emission peaking at 497 nm with biexponential decay pattern of τ1 = 5.5414 μs (A1 = 0.137) and τ2 = 1.0679 μs (A2 = 0.11503). Cyclic voltammerty experiment suggested that this Cu(I) complex owned HOMO and LUMO energy levels of −5.79 eV and −2.39 eV. The thermal decomposition temperature was 170 °C as indicated by thermogravimetric analysis. The optimal electroluminescence device constructed by solution processed coating procedure showed green electroluminescence peaking at 525 nm, with maximum luminance of 2860 cd/m2 and maximum current efficiency of 5.9 cd/A.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide