| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230354 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 8 Pages |
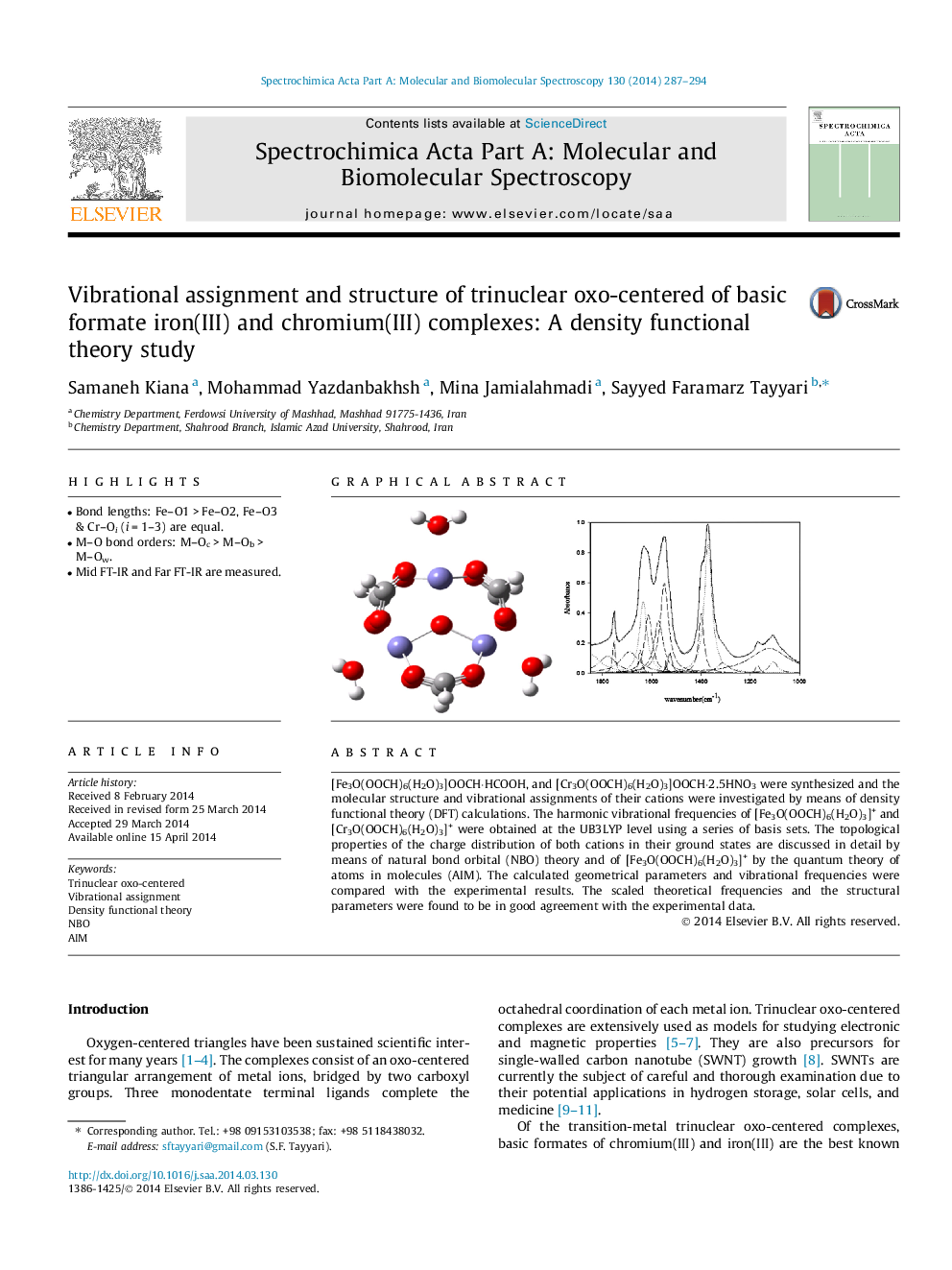
•Bond lengths: Fe–O1 > Fe–O2, Fe–O3 & Cr–Oi (i = 1–3) are equal.•M–O bond orders: M–Oc > M–Ob > M–Ow.•Mid FT-IR and Far FT-IR are measured.
[Fe3O(OOCH)6(H2O)3]OOCH·HCOOH, and [Cr3O(OOCH)6(H2O)3]OOCH·2.5HNO3 were synthesized and the molecular structure and vibrational assignments of their cations were investigated by means of density functional theory (DFT) calculations. The harmonic vibrational frequencies of [Fe3O(OOCH)6(H2O)3]+ and [Cr3O(OOCH)6(H2O)3]+ were obtained at the UB3LYP level using a series of basis sets. The topological properties of the charge distribution of both cations in their ground states are discussed in detail by means of natural bond orbital (NBO) theory and of [Fe3O(OOCH)6(H2O)3]+ by the quantum theory of atoms in molecules (AIM). The calculated geometrical parameters and vibrational frequencies were compared with the experimental results. The scaled theoretical frequencies and the structural parameters were found to be in good agreement with the experimental data.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide