| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230357 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 4 Pages |
•Nanostructure copper oxocobaltate was fabricated by co-precipitation.•Copper and cobalt nitrate were used as precursors.•FTIR and UV-DRS measurements were performed for structural and optical properties.
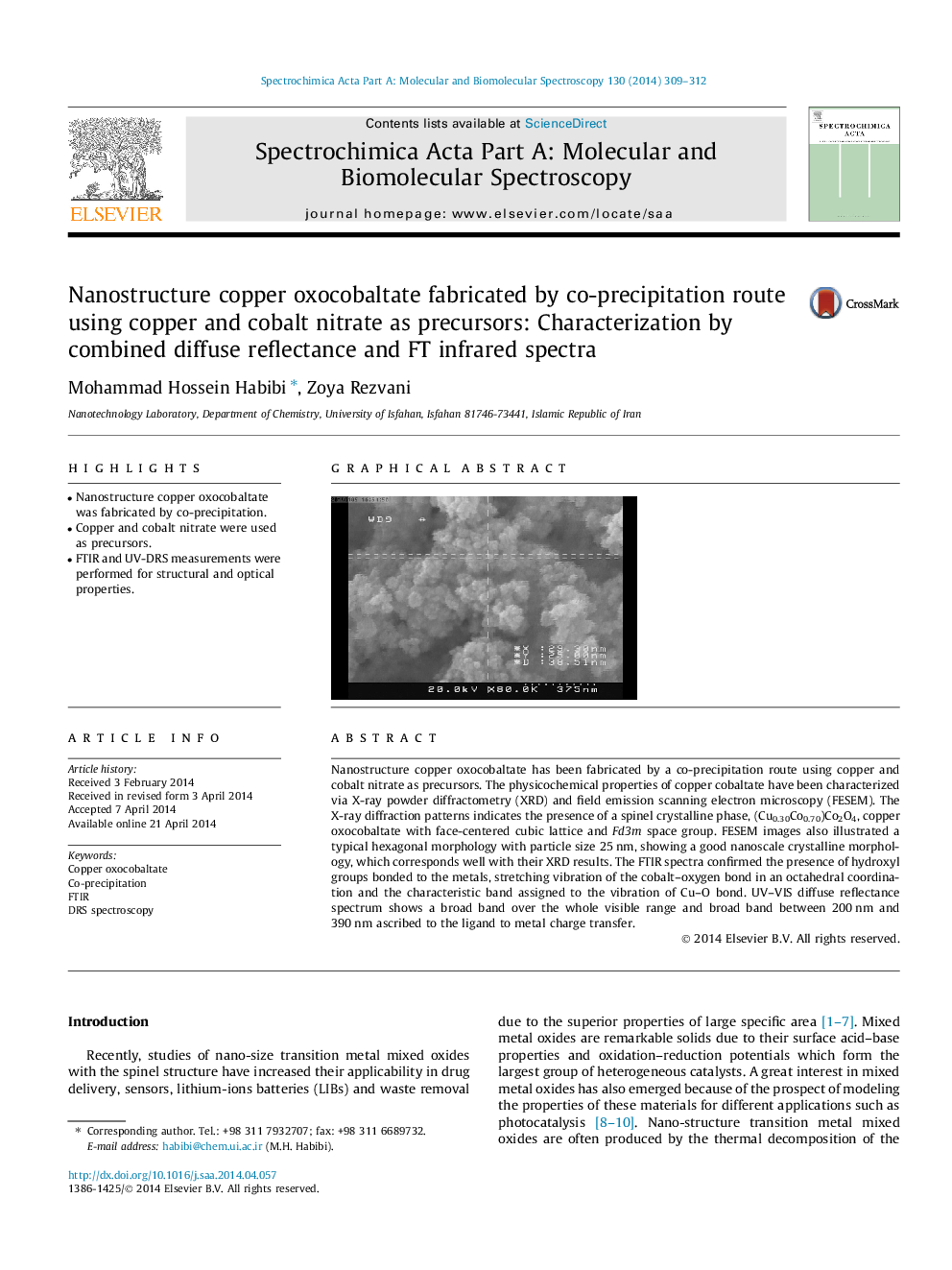
Nanostructure copper oxocobaltate has been fabricated by a co-precipitation route using copper and cobalt nitrate as precursors. The physicochemical properties of copper cobaltate have been characterized via X-ray powder diffractometry (XRD) and field emission scanning electron microscopy (FESEM). The X-ray diffraction patterns indicates the presence of a spinel crystalline phase, (Cu0.30Co0.70)Co2O4, copper oxocobaltate with face-centered cubic lattice and Fd3m space group. FESEM images also illustrated a typical hexagonal morphology with particle size 25 nm, showing a good nanoscale crystalline morphology, which corresponds well with their XRD results. The FTIR spectra confirmed the presence of hydroxyl groups bonded to the metals, stretching vibration of the cobalt–oxygen bond in an octahedral coordination and the characteristic band assigned to the vibration of Cu–O bond. UV–VIS diffuse reflectance spectrum shows a broad band over the whole visible range and broad band between 200 nm and 390 nm ascribed to the ligand to metal charge transfer.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide