| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230358 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 16 Pages |
•IR and Raman spectra of uracil and 5-aminouracil bio-molecules were recorded and analyzed.•Vibrational frequencies were calculated at DFT–B3LYP/6-311++G** level of Gaussian-03.•Electro-negativity on C5 atom is found to be wide-variation from −ve to +ve due to amino (NH2) group.•Kekule-ring mode is observed higher than uracil due to the hydrogen bonding of amino group.•Due to amino (NH2) group, ring-breathing is observed lower than uracil.
Infrared (IR) and Raman spectra of uracil and 5-aminouracil have been recorded and analyzed between the region 200–4000 cm−1. The optimized molecular geometries, atomic polar tensor (APT) charges and vibrational characteristics have been studied theoretically using restricted Hartree–Fock (RHF) and density functional theory (DFT) methods. Using the Becke’s exchange in conjunction with Lee–Yang–Parr’s correlation functional and Becke’s three-parameter hybrid method (B3LYP), the ab initio and DFT calculations were carried out to study the optimized molecular fundamental vibrational frequencies for uracil and 5-aminouracil by employing Gaussian-03 program. The fundamental vibrational frequencies along with their corresponding intensities in IR and Raman activities and depolarization ratios of the Raman lines have also been calculated using the RHF and DFT methods employing different basis sets. In quantum chemical calculations, the most of B3LYP/6-311++G** vibrational frequencies are in the excellent agreement with available experimental assignments and helped in the reassignments of some fundamental vibrational modes. On the basis of calculated results, the assignments of some missing frequencies in the experimental study are proposed. Assuming under the Cs point group for both molecules, the distribution of normal mode of vibrations between the two species as planar (a′) and non-planar (a″) are given by 25a′ + 11a″, of which 30 modes (21a′ + 9a″) correspond to the uracil moiety and 6 modes (4a′ + 2a″) to the NH2 group. Kekule ring stretching mode is found to be comparatively higher frequency magnitude than the mode of uracil due to the involvement of hydrogen bonding of amino group. But, the ring breathing is found to be lower frequency magnitude compared to those for uracil which could be due to mass effect of the NH2 group in place of the hydrogen atom. All other bands have also been assigned different fundamentals/overtones/combinations.
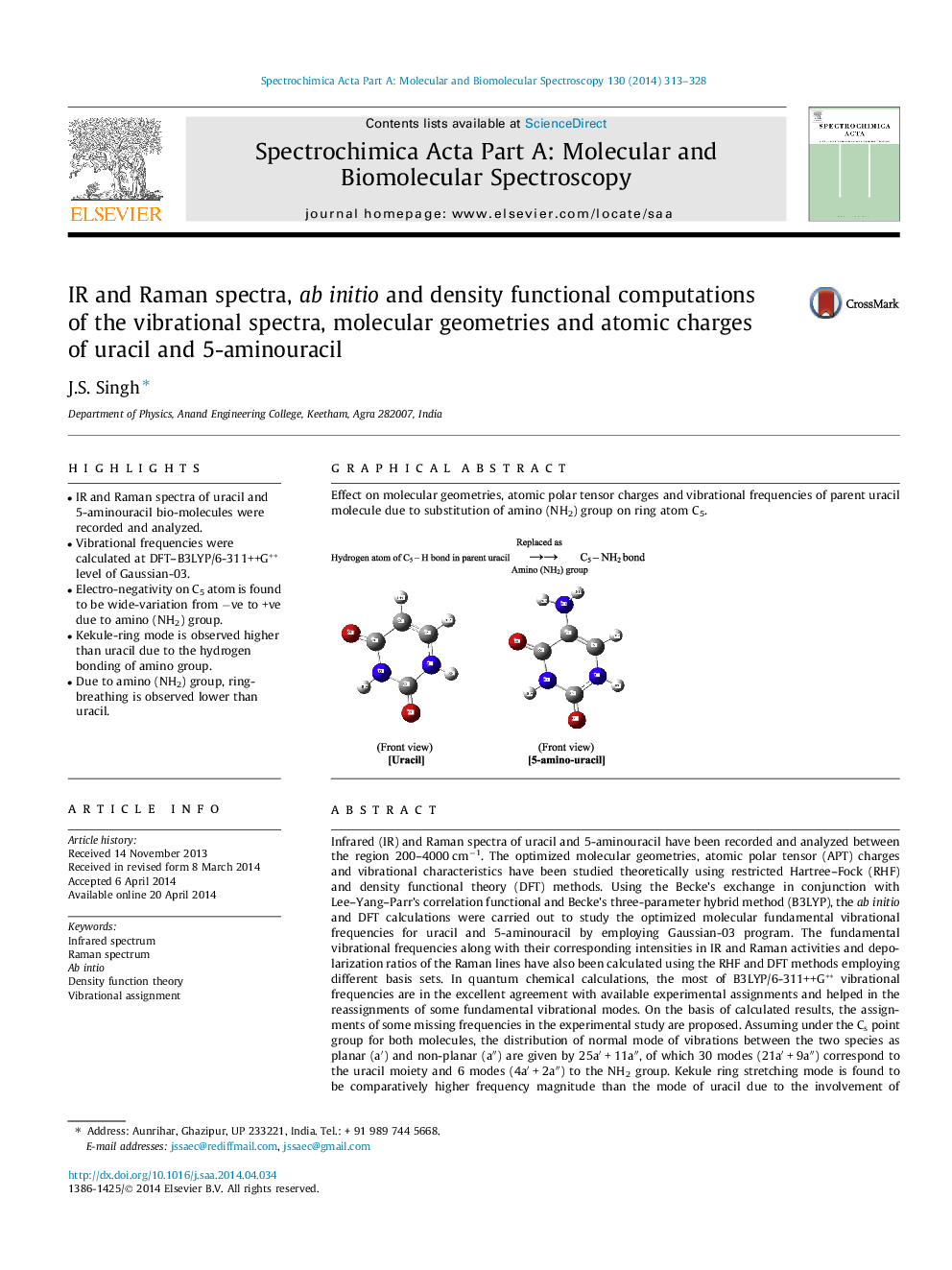
Graphical abstractEffect on molecular geometries, atomic polar tensor charges and vibrational frequencies of parent uracil molecule due to substitution of amino (NH2) group on ring atom C5.Figure optionsDownload full-size imageDownload as PowerPoint slide