| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230378 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 8 Pages |
•This study includes rapid stability indicating HPLC determination of acrivastine.•As stationary phase monolithic column was used at flow rate of 5.0 mL/min.•Separation of acrivastine from its degradants took place in less than 3.0 min.•Also two spectrophotometric methods were represented.•Oxidation of acrivastine with excess N-bromosuccinimide.
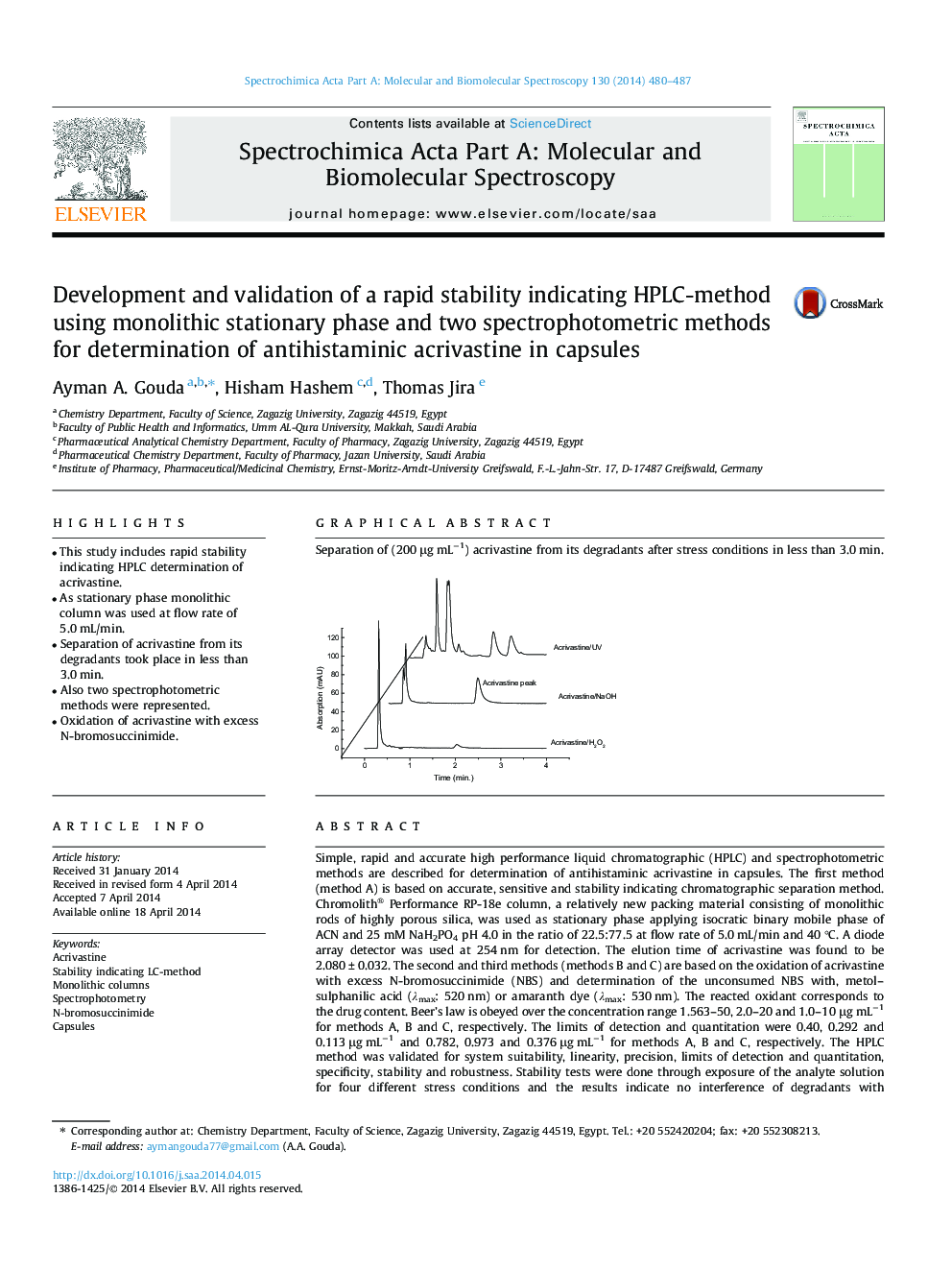
Simple, rapid and accurate high performance liquid chromatographic (HPLC) and spectrophotometric methods are described for determination of antihistaminic acrivastine in capsules. The first method (method A) is based on accurate, sensitive and stability indicating chromatographic separation method. Chromolith® Performance RP-18e column, a relatively new packing material consisting of monolithic rods of highly porous silica, was used as stationary phase applying isocratic binary mobile phase of ACN and 25 mM NaH2PO4 pH 4.0 in the ratio of 22.5:77.5 at flow rate of 5.0 mL/min and 40 °C. A diode array detector was used at 254 nm for detection. The elution time of acrivastine was found to be 2.080 ± 0.032. The second and third methods (methods B and C) are based on the oxidation of acrivastine with excess N-bromosuccinimide (NBS) and determination of the unconsumed NBS with, metol–sulphanilic acid (λmax: 520 nm) or amaranth dye (λmax: 530 nm). The reacted oxidant corresponds to the drug content. Beer’s law is obeyed over the concentration range 1.563–50, 2.0–20 and 1.0–10 μg mL−1 for methods A, B and C, respectively. The limits of detection and quantitation were 0.40, 0.292 and 0.113 μg mL−1 and 0.782, 0.973 and 0.376 μg mL−1 for methods A, B and C, respectively. The HPLC method was validated for system suitability, linearity, precision, limits of detection and quantitation, specificity, stability and robustness. Stability tests were done through exposure of the analyte solution for four different stress conditions and the results indicate no interference of degradants with HPLC-method. The proposed methods was favorably applied for determination of acrivastine in capsules formulation. Statistical comparison of the obtained results from the analysis of the studied drug to those of the reported method using t- and F-tests showed no significant difference between them.
Graphical abstractSeparation of (200 μg mL−1) acrivastine from its degradants after stress conditions in less than 3.0 min.Figure optionsDownload full-size imageDownload as PowerPoint slide