| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230387 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 8 Pages |
•Binding of ruthenium(II) complexes is efficient with BSA.•Sensing of ruthenium(II) complexes with BSA and HSA is higher than other proteins.•The α helicity of BSA has decreased with the addition of ruthenium(II) complexes.•Mode of binding is established by docking studies.
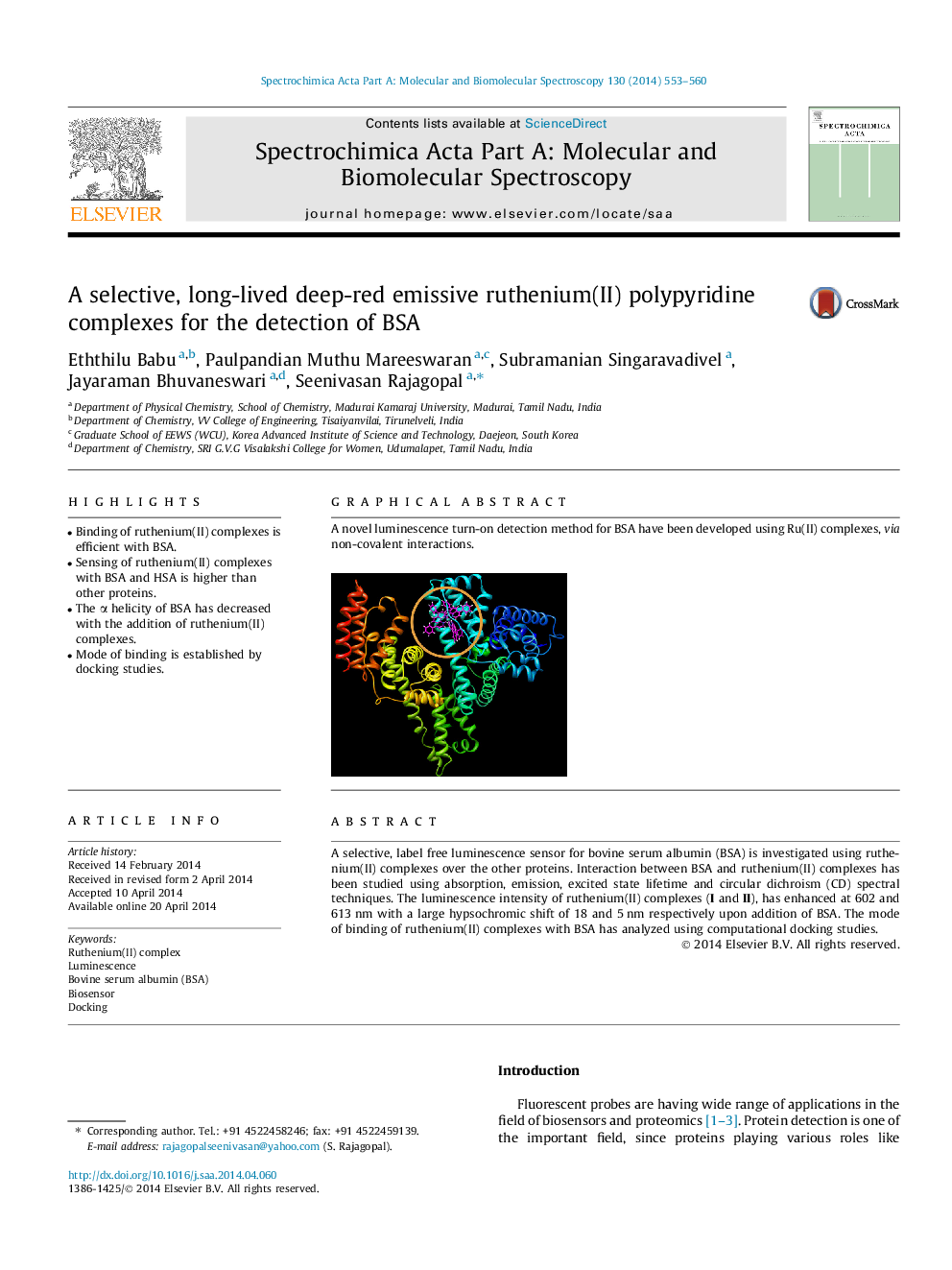
A selective, label free luminescence sensor for bovine serum albumin (BSA) is investigated using ruthenium(II) complexes over the other proteins. Interaction between BSA and ruthenium(II) complexes has been studied using absorption, emission, excited state lifetime and circular dichroism (CD) spectral techniques. The luminescence intensity of ruthenium(II) complexes (I and II), has enhanced at 602 and 613 nm with a large hypsochromic shift of 18 and 5 nm respectively upon addition of BSA. The mode of binding of ruthenium(II) complexes with BSA has analyzed using computational docking studies.
Graphical abstractA novel luminescence turn-on detection method for BSA have been developed using Ru(II) complexes, via non-covalent interactions.Figure optionsDownload full-size imageDownload as PowerPoint slide