| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230461 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 7 Pages |
•The first report of dispersive liquid–liquid microextraction for an anionic species.•Trace levels of iodide was determined spectrophotometrically in water samples.•Advantages: simple, rapid, low sample volume, economical, high enrichment factor.•Be useful in less developed area with no access to advanced analytical instruments.
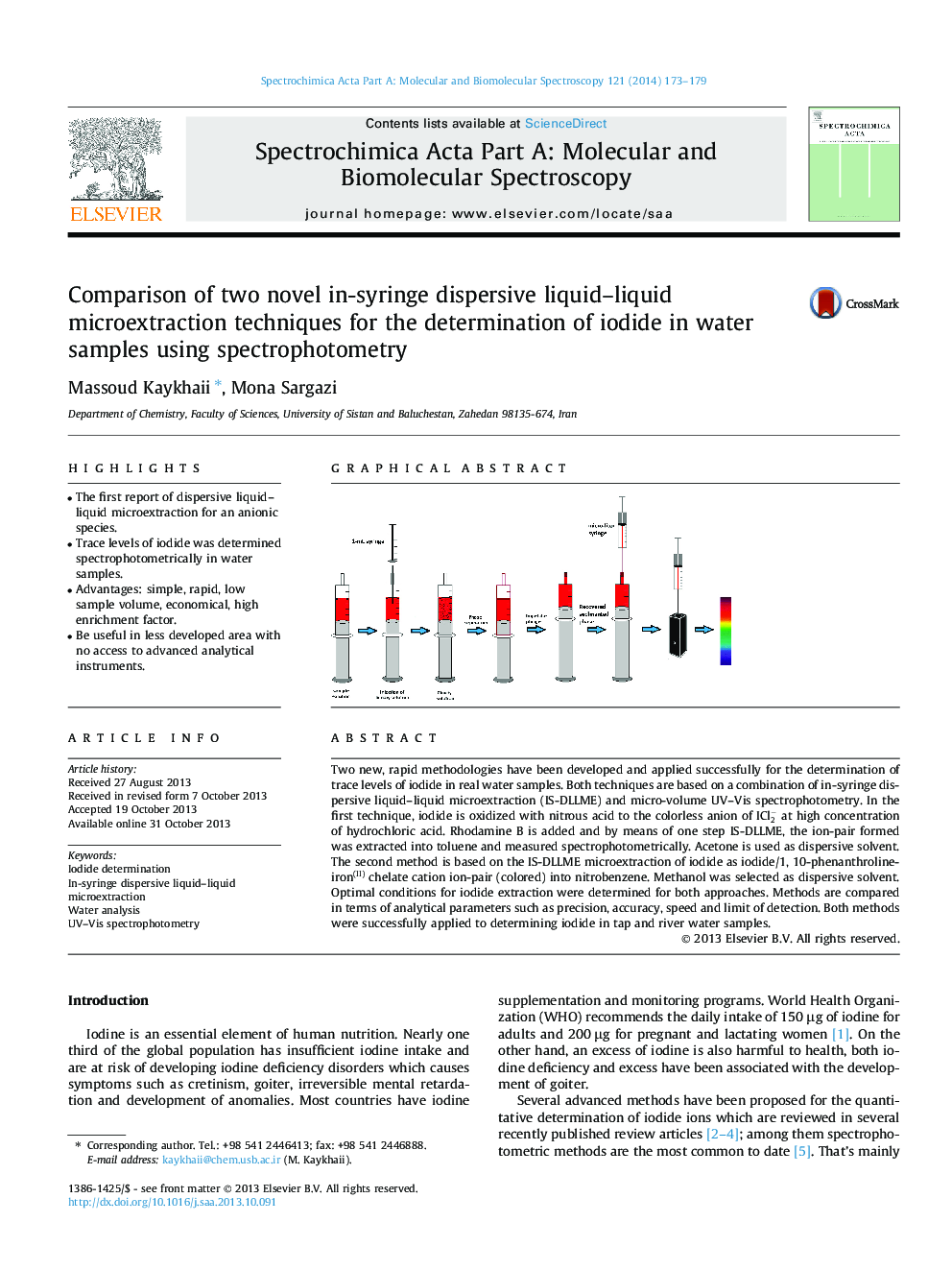
Two new, rapid methodologies have been developed and applied successfully for the determination of trace levels of iodide in real water samples. Both techniques are based on a combination of in-syringe dispersive liquid–liquid microextraction (IS-DLLME) and micro-volume UV–Vis spectrophotometry. In the first technique, iodide is oxidized with nitrous acid to the colorless anion of ICl2- at high concentration of hydrochloric acid. Rhodamine B is added and by means of one step IS-DLLME, the ion-pair formed was extracted into toluene and measured spectrophotometrically. Acetone is used as dispersive solvent. The second method is based on the IS-DLLME microextraction of iodide as iodide/1, 10-phenanthroline-iron(II) chelate cation ion-pair (colored) into nitrobenzene. Methanol was selected as dispersive solvent. Optimal conditions for iodide extraction were determined for both approaches. Methods are compared in terms of analytical parameters such as precision, accuracy, speed and limit of detection. Both methods were successfully applied to determining iodide in tap and river water samples.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide