| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230546 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 9 Pages |
•The FT-IR and Laser-Raman spectra of the title compound were recorded in solid phase.•The optimized geometry and vibrational frequencies were calculated for the first time.•The wavenumbers are assigned using PED analysis.•The HOMO–LUMO energies and related molecular properties were evaluated.
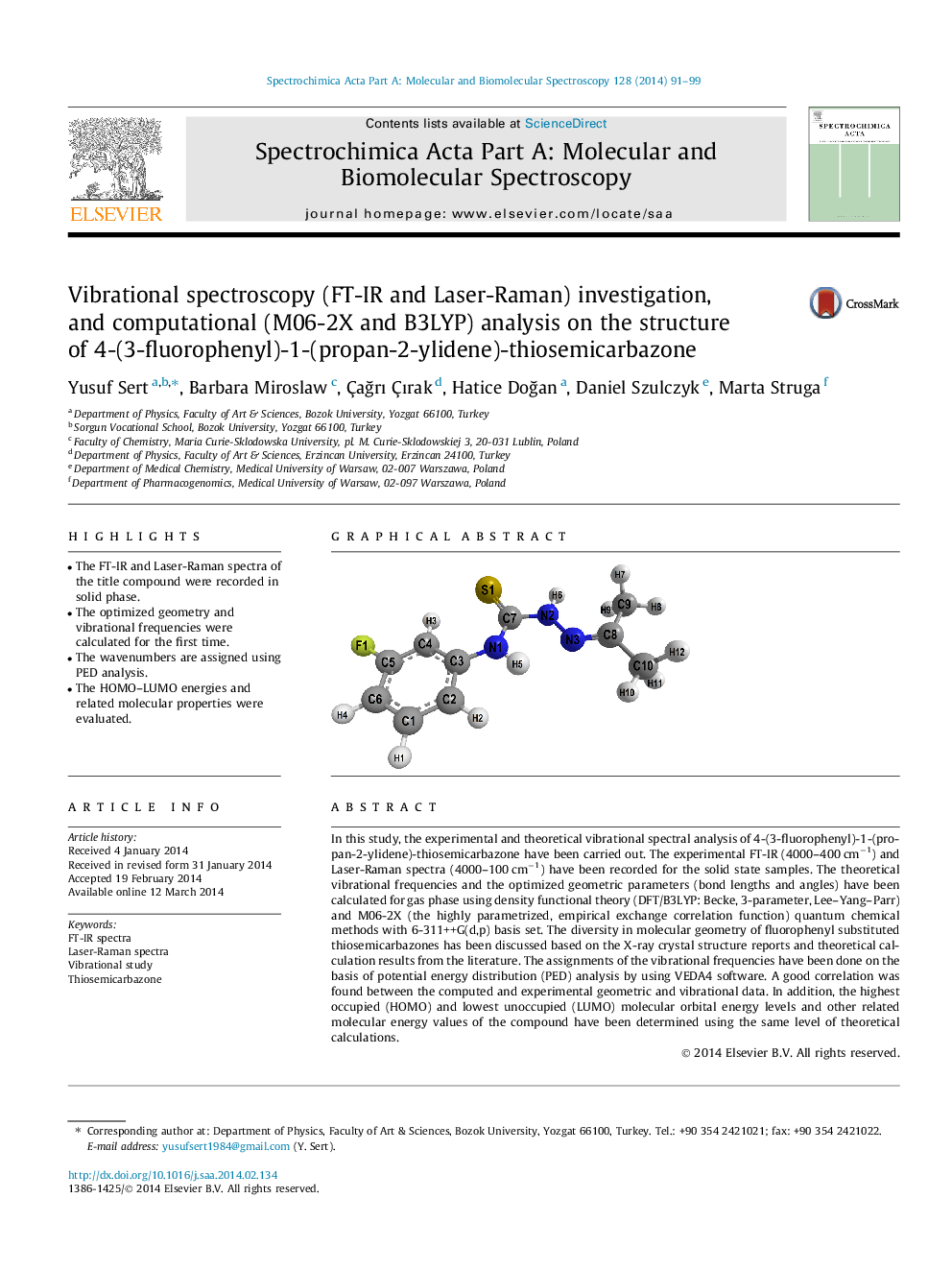
In this study, the experimental and theoretical vibrational spectral analysis of 4-(3-fluorophenyl)-1-(propan-2-ylidene)-thiosemicarbazone have been carried out. The experimental FT-IR (4000–400 cm−1) and Laser-Raman spectra (4000–100 cm−1) have been recorded for the solid state samples. The theoretical vibrational frequencies and the optimized geometric parameters (bond lengths and angles) have been calculated for gas phase using density functional theory (DFT/B3LYP: Becke, 3-parameter, Lee–Yang–Parr) and M06-2X (the highly parametrized, empirical exchange correlation function) quantum chemical methods with 6-311++G(d,p) basis set. The diversity in molecular geometry of fluorophenyl substituted thiosemicarbazones has been discussed based on the X-ray crystal structure reports and theoretical calculation results from the literature. The assignments of the vibrational frequencies have been done on the basis of potential energy distribution (PED) analysis by using VEDA4 software. A good correlation was found between the computed and experimental geometric and vibrational data. In addition, the highest occupied (HOMO) and lowest unoccupied (LUMO) molecular orbital energy levels and other related molecular energy values of the compound have been determined using the same level of theoretical calculations.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide