| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230553 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
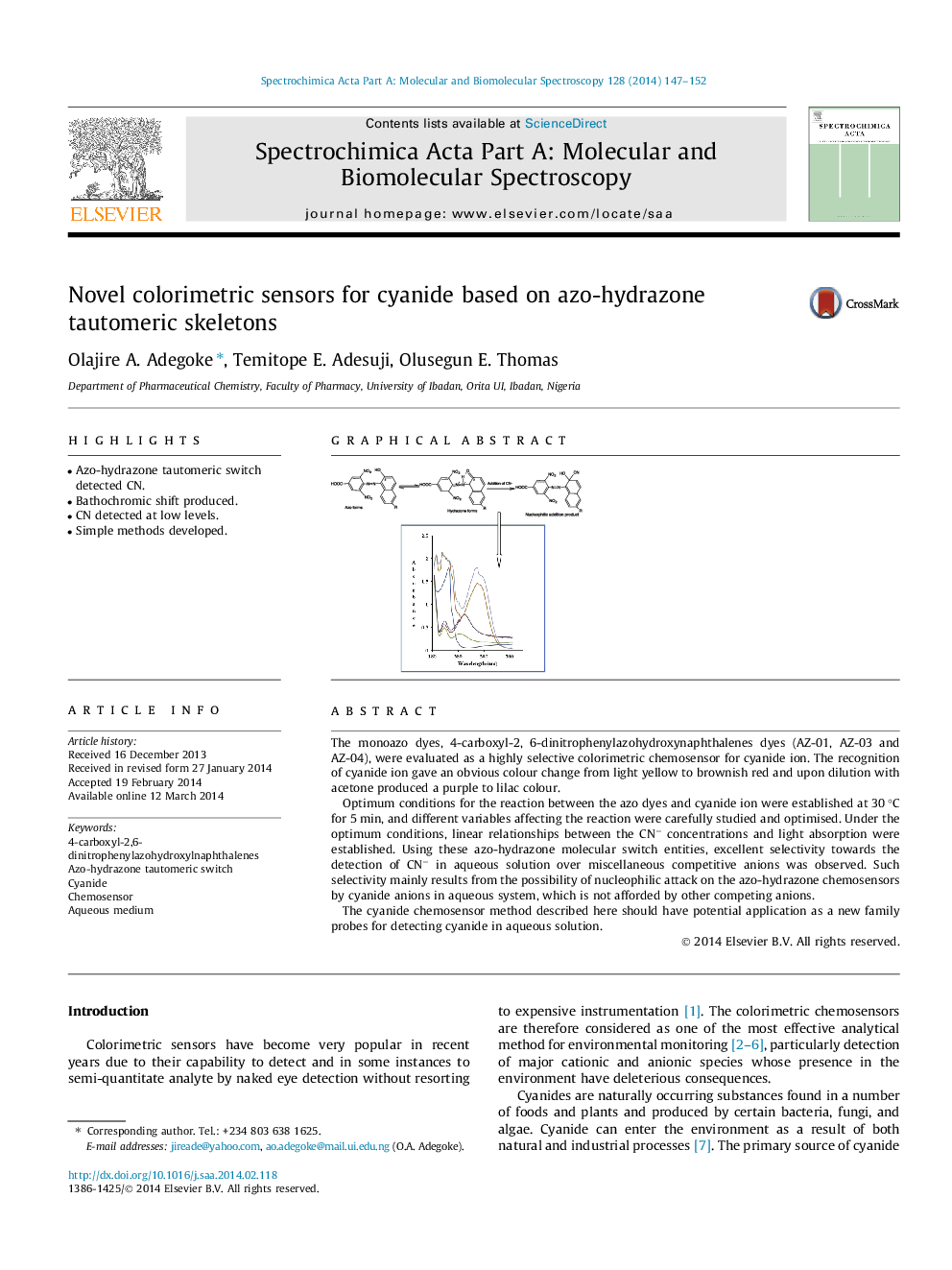
•Azo-hydrazone tautomeric switch detected CN.•Bathochromic shift produced.•CN detected at low levels.•Simple methods developed.
The monoazo dyes, 4-carboxyl-2, 6-dinitrophenylazohydroxynaphthalenes dyes (AZ-01, AZ-03 and AZ-04), were evaluated as a highly selective colorimetric chemosensor for cyanide ion. The recognition of cyanide ion gave an obvious colour change from light yellow to brownish red and upon dilution with acetone produced a purple to lilac colour.Optimum conditions for the reaction between the azo dyes and cyanide ion were established at 30 °C for 5 min, and different variables affecting the reaction were carefully studied and optimised. Under the optimum conditions, linear relationships between the CN− concentrations and light absorption were established. Using these azo-hydrazone molecular switch entities, excellent selectivity towards the detection of CN− in aqueous solution over miscellaneous competitive anions was observed. Such selectivity mainly results from the possibility of nucleophilic attack on the azo-hydrazone chemosensors by cyanide anions in aqueous system, which is not afforded by other competing anions.The cyanide chemosensor method described here should have potential application as a new family probes for detecting cyanide in aqueous solution.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide