| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230556 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 8 Pages |
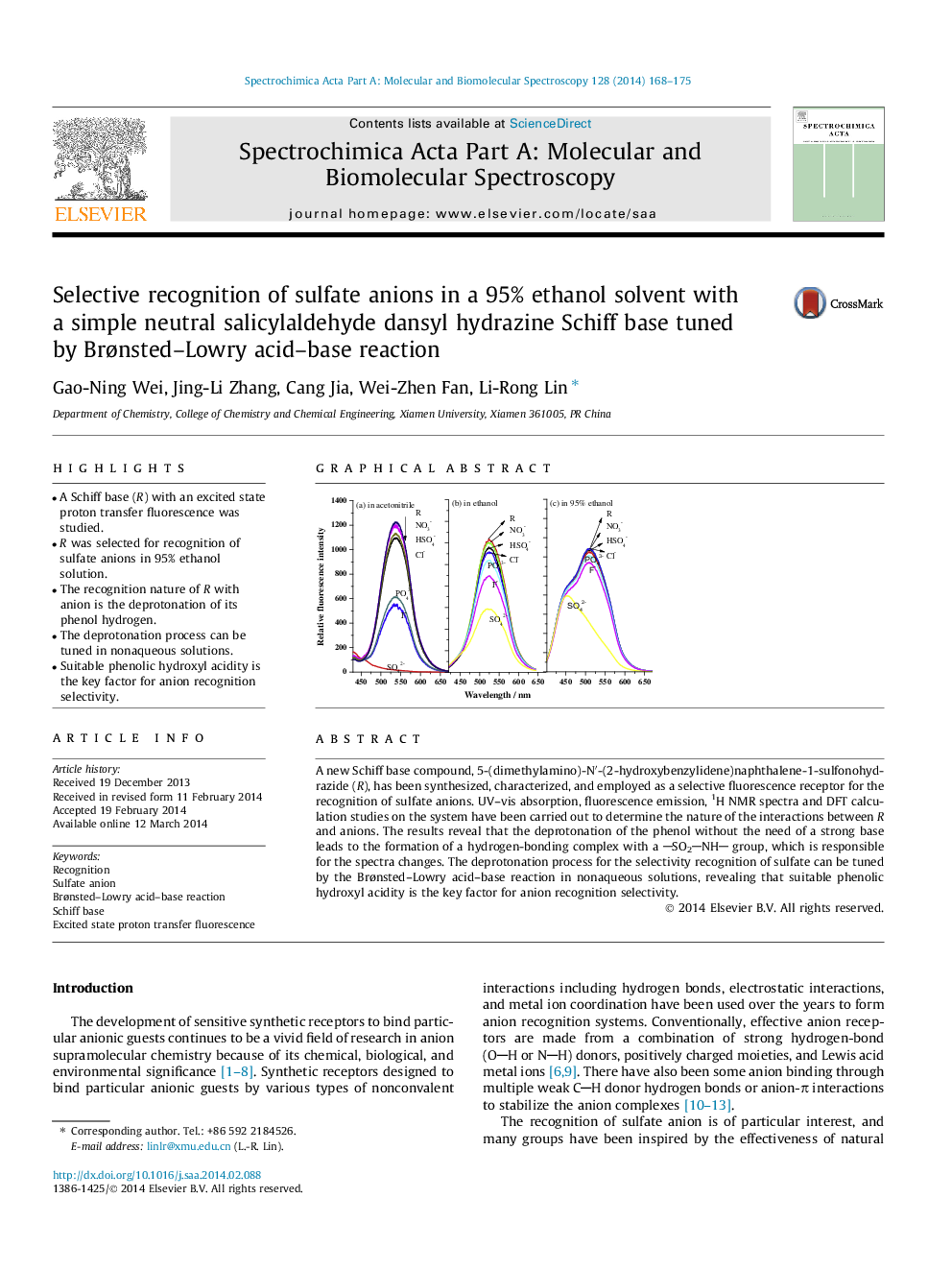
•A Schiff base (R) with an excited state proton transfer fluorescence was studied.•R was selected for recognition of sulfate anions in 95% ethanol solution.•The recognition nature of R with anion is the deprotonation of its phenol hydrogen.•The deprotonation process can be tuned in nonaqueous solutions.•Suitable phenolic hydroxyl acidity is the key factor for anion recognition selectivity.
A new Schiff base compound, 5-(dimethylamino)-N′-(2-hydroxybenzylidene)naphthalene-1-sulfonohydrazide (R), has been synthesized, characterized, and employed as a selective fluorescence receptor for the recognition of sulfate anions. UV–vis absorption, fluorescence emission, 1H NMR spectra and DFT calculation studies on the system have been carried out to determine the nature of the interactions between R and anions. The results reveal that the deprotonation of the phenol without the need of a strong base leads to the formation of a hydrogen-bonding complex with a SO2NH group, which is responsible for the spectra changes. The deprotonation process for the selectivity recognition of sulfate can be tuned by the Brønsted–Lowry acid–base reaction in nonaqueous solutions, revealing that suitable phenolic hydroxyl acidity is the key factor for anion recognition selectivity.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide