| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230566 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 5 Pages |
•We synthesized the macrocyclic complexes by template method in presence of divalent transition metal ions.•Complexes were characterized by various spectral techniques, such as IR, NMR, EPR, and electronic.•Distorted octahedral geometry has been proposed for the complexes.•In vitro antibacterial activities were reported.
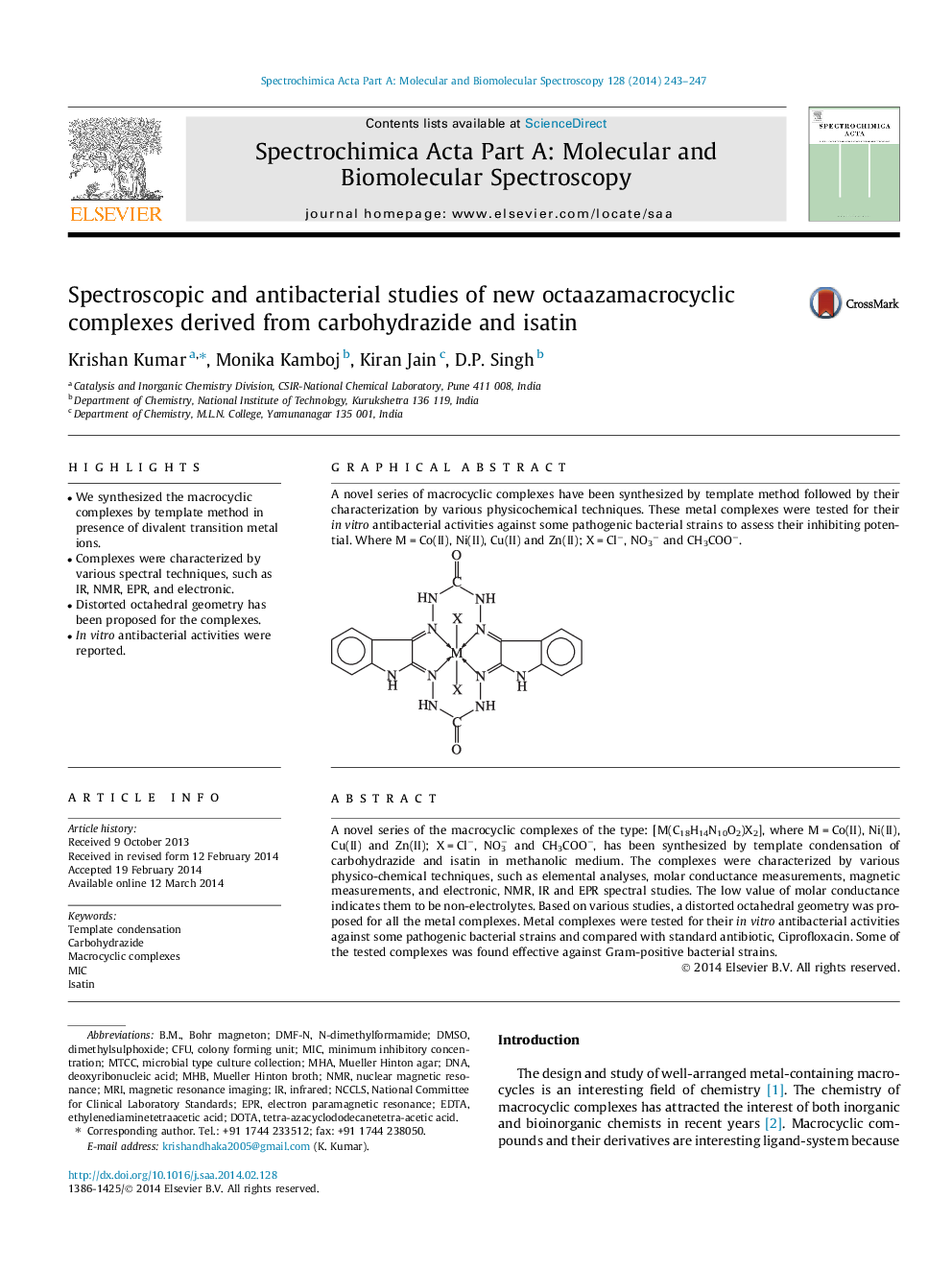
A novel series of the macrocyclic complexes of the type: [M(C18H14N10O2)X2], where M = Co(II), Ni(II), Cu(II) and Zn(II); X = Cl−, NO3− and CH3COO−, has been synthesized by template condensation of carbohydrazide and isatin in methanolic medium. The complexes were characterized by various physico-chemical techniques, such as elemental analyses, molar conductance measurements, magnetic measurements, and electronic, NMR, IR and EPR spectral studies. The low value of molar conductance indicates them to be non-electrolytes. Based on various studies, a distorted octahedral geometry was proposed for all the metal complexes. Metal complexes were tested for their in vitro antibacterial activities against some pathogenic bacterial strains and compared with standard antibiotic, Ciprofloxacin. Some of the tested complexes was found effective against Gram-positive bacterial strains.
Graphical abstractA novel series of macrocyclic complexes have been synthesized by template method followed by their characterization by various physicochemical techniques. These metal complexes were tested for their in vitro antibacterial activities against some pathogenic bacterial strains to assess their inhibiting potential. Where M = Co(II), Ni(II), Cu(II) and Zn(II); X = Cl−, NO3− and CH3COO−.Figure optionsDownload full-size imageDownload as PowerPoint slide