| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230570 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 8 Pages |
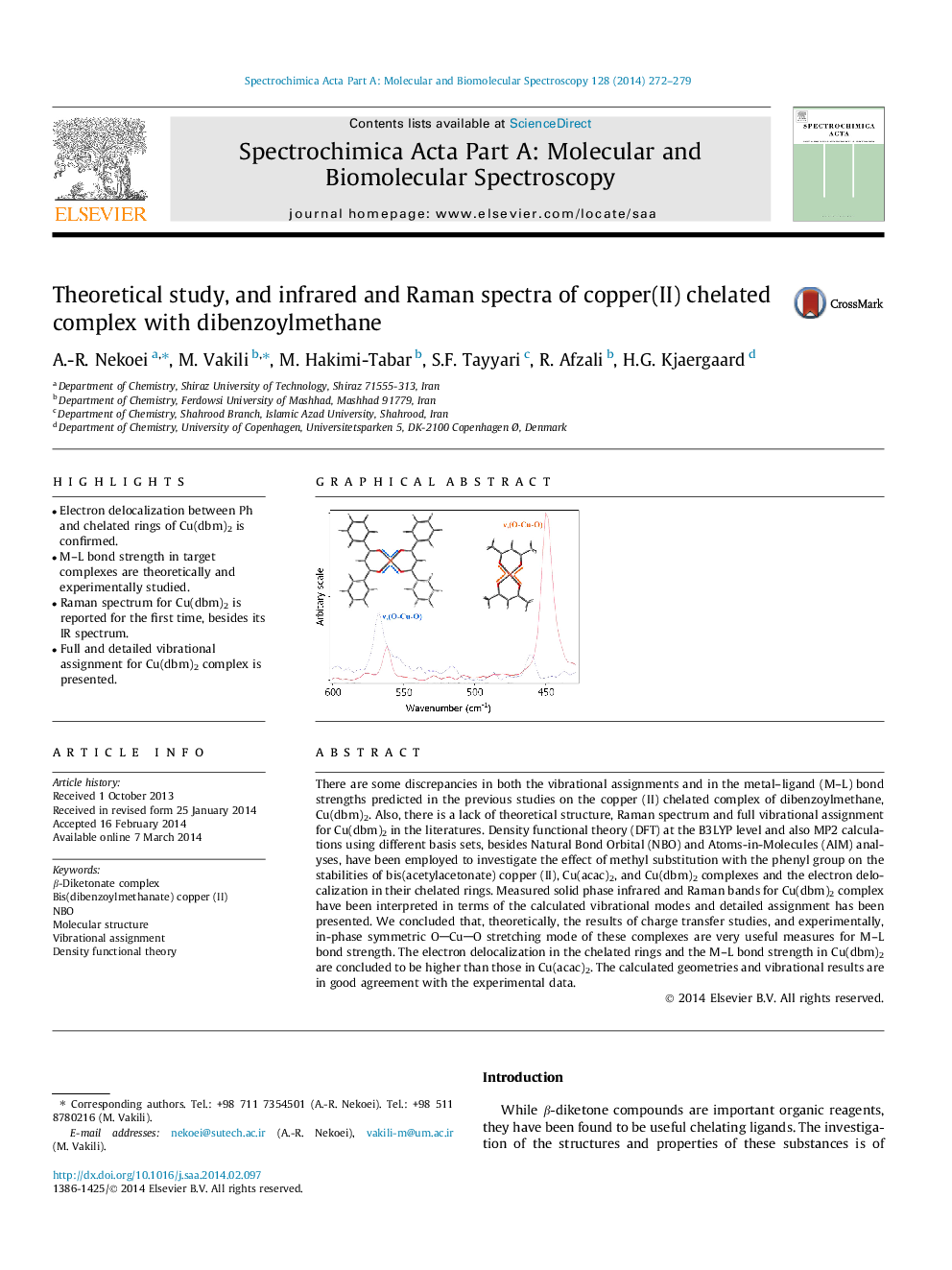
•Electron delocalization between Ph and chelated rings of Cu(dbm)2 is confirmed.•M–L bond strength in target complexes are theoretically and experimentally studied.•Raman spectrum for Cu(dbm)2 is reported for the first time, besides its IR spectrum.•Full and detailed vibrational assignment for Cu(dbm)2 complex is presented.
There are some discrepancies in both the vibrational assignments and in the metal–ligand (M–L) bond strengths predicted in the previous studies on the copper (II) chelated complex of dibenzoylmethane, Cu(dbm)2. Also, there is a lack of theoretical structure, Raman spectrum and full vibrational assignment for Cu(dbm)2 in the literatures. Density functional theory (DFT) at the B3LYP level and also MP2 calculations using different basis sets, besides Natural Bond Orbital (NBO) and Atoms-in-Molecules (AIM) analyses, have been employed to investigate the effect of methyl substitution with the phenyl group on the stabilities of bis(acetylacetonate) copper (II), Cu(acac)2, and Cu(dbm)2 complexes and the electron delocalization in their chelated rings. Measured solid phase infrared and Raman bands for Cu(dbm)2 complex have been interpreted in terms of the calculated vibrational modes and detailed assignment has been presented. We concluded that, theoretically, the results of charge transfer studies, and experimentally, in-phase symmetric OCuO stretching mode of these complexes are very useful measures for M–L bond strength. The electron delocalization in the chelated rings and the M–L bond strength in Cu(dbm)2 are concluded to be higher than those in Cu(acac)2. The calculated geometries and vibrational results are in good agreement with the experimental data.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide