| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230597 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 7 Pages |
•Nano TiO2 and TiO2–SiO2 composites were successfully synthesized by sol–gel method.•Acid red 88 was degraded by Nano TiO2 and TiO2–SiO2 composites using solar energy.•TiO2 nanoparticles and TiO2–SiO2 nanocomposites are good photo catalysts.
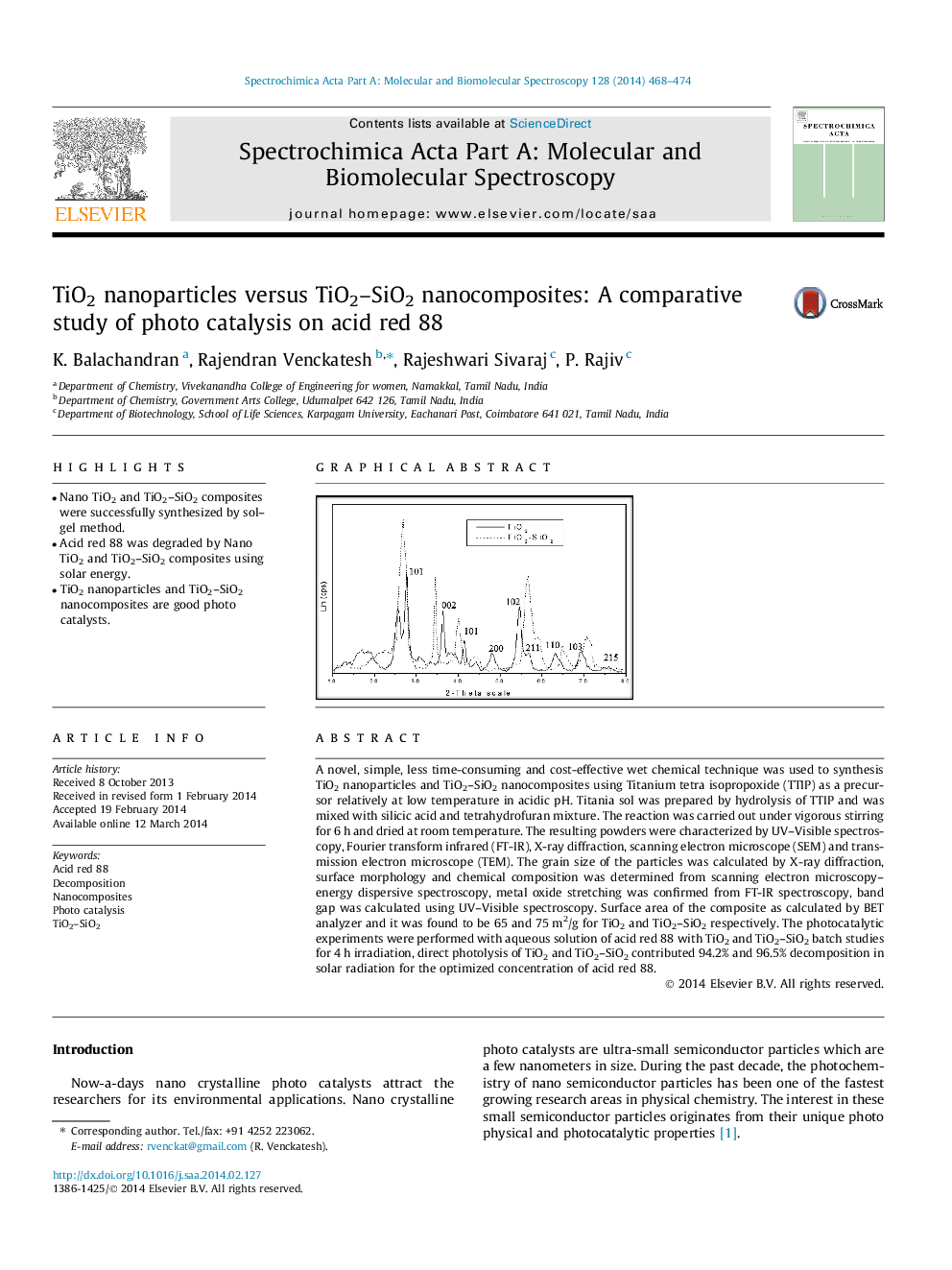
A novel, simple, less time-consuming and cost-effective wet chemical technique was used to synthesis TiO2 nanoparticles and TiO2–SiO2 nanocomposites using Titanium tetra isopropoxide (TTIP) as a precursor relatively at low temperature in acidic pH. Titania sol was prepared by hydrolysis of TTIP and was mixed with silicic acid and tetrahydrofuran mixture. The reaction was carried out under vigorous stirring for 6 h and dried at room temperature. The resulting powders were characterized by UV–Visible spectroscopy, Fourier transform infrared (FT-IR), X-ray diffraction, scanning electron microscope (SEM) and transmission electron microscope (TEM). The grain size of the particles was calculated by X-ray diffraction, surface morphology and chemical composition was determined from scanning electron microscopy–energy dispersive spectroscopy, metal oxide stretching was confirmed from FT-IR spectroscopy, band gap was calculated using UV–Visible spectroscopy. Surface area of the composite as calculated by BET analyzer and it was found to be 65 and 75 m2/g for TiO2 and TiO2–SiO2 respectively. The photocatalytic experiments were performed with aqueous solution of acid red 88 with TiO2 and TiO2–SiO2 batch studies for 4 h irradiation, direct photolysis of TiO2 and TiO2–SiO2 contributed 94.2% and 96.5% decomposition in solar radiation for the optimized concentration of acid red 88.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide