| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230712 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 6 Pages |
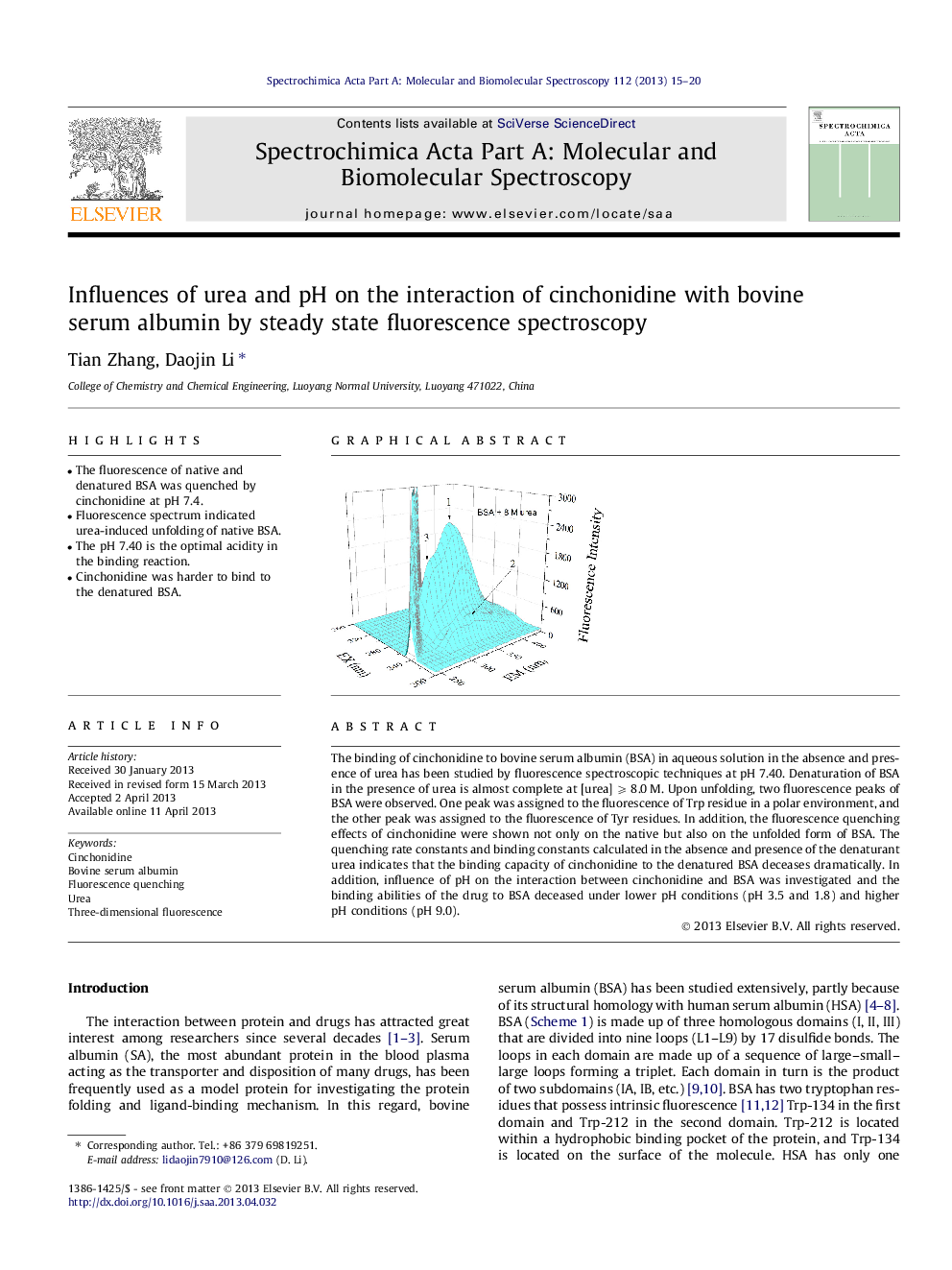
•The fluorescence of native and denatured BSA was quenched by cinchonidine at pH 7.4.•Fluorescence spectrum indicated urea-induced unfolding of native BSA.•The pH 7.40 is the optimal acidity in the binding reaction.•Cinchonidine was harder to bind to the denatured BSA.
The binding of cinchonidine to bovine serum albumin (BSA) in aqueous solution in the absence and presence of urea has been studied by fluorescence spectroscopic techniques at pH 7.40. Denaturation of BSA in the presence of urea is almost complete at [urea] ⩾ 8.0 M. Upon unfolding, two fluorescence peaks of BSA were observed. One peak was assigned to the fluorescence of Trp residue in a polar environment, and the other peak was assigned to the fluorescence of Tyr residues. In addition, the fluorescence quenching effects of cinchonidine were shown not only on the native but also on the unfolded form of BSA. The quenching rate constants and binding constants calculated in the absence and presence of the denaturant urea indicates that the binding capacity of cinchonidine to the denatured BSA deceases dramatically. In addition, influence of pH on the interaction between cinchonidine and BSA was investigated and the binding abilities of the drug to BSA deceased under lower pH conditions (pH 3.5 and 1.8) and higher pH conditions (pH 9.0).
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide