| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230719 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 6 Pages |
•Ferulic acid could insert into DNA base pairs to form a binary complex at physiological pH.•The acting forces between ferulic acid and DNA mainly included hydrophobic interactions and hydrogen bonds.•Ferulic acid could substitute for AO probe in the AO-DNA complex by intercalation mode.•The stabilization of the ctDNA helix was increased in the presence of ferulic acid.•The interaction between ferulic acid and ctDNA might occur via intercalative mode.
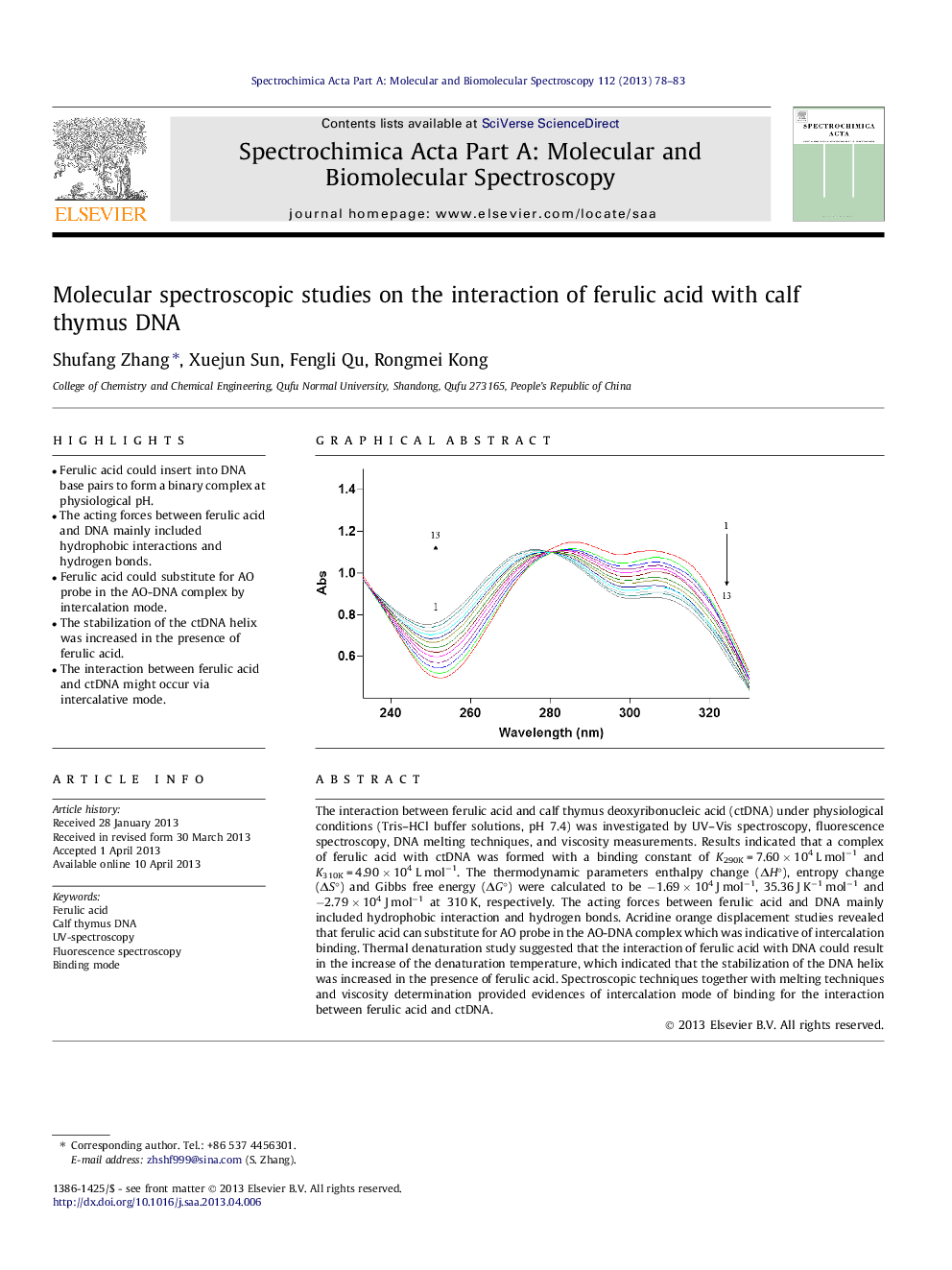
The interaction between ferulic acid and calf thymus deoxyribonucleic acid (ctDNA) under physiological conditions (Tris–HCl buffer solutions, pH 7.4) was investigated by UV–Vis spectroscopy, fluorescence spectroscopy, DNA melting techniques, and viscosity measurements. Results indicated that a complex of ferulic acid with ctDNA was formed with a binding constant of K290K = 7.60 × 104 L mol−1 and K310K = 4.90 × 104 L mol−1. The thermodynamic parameters enthalpy change (ΔH°), entropy change (ΔS°) and Gibbs free energy (ΔG°) were calculated to be −1.69 × 104 J mol−1, 35.36 J K−1 mol−1 and −2.79 × 104 J mol−1 at 310 K, respectively. The acting forces between ferulic acid and DNA mainly included hydrophobic interaction and hydrogen bonds. Acridine orange displacement studies revealed that ferulic acid can substitute for AO probe in the AO-DNA complex which was indicative of intercalation binding. Thermal denaturation study suggested that the interaction of ferulic acid with DNA could result in the increase of the denaturation temperature, which indicated that the stabilization of the DNA helix was increased in the presence of ferulic acid. Spectroscopic techniques together with melting techniques and viscosity determination provided evidences of intercalation mode of binding for the interaction between ferulic acid and ctDNA.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide