| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230720 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 6 Pages |
•Reaction of urea with thiosemicarbazide is reinvestigated.•Urea being an amide does not form any thiosemicarbazone product.•Thiosemicarbazide crystallizes in the triclinic P1¯ space group.•Thiosemicarbazide exhibits thione-thiol tautomerism in solution.•Thiosemicarbazide exists as the thione tautomer in solid state.
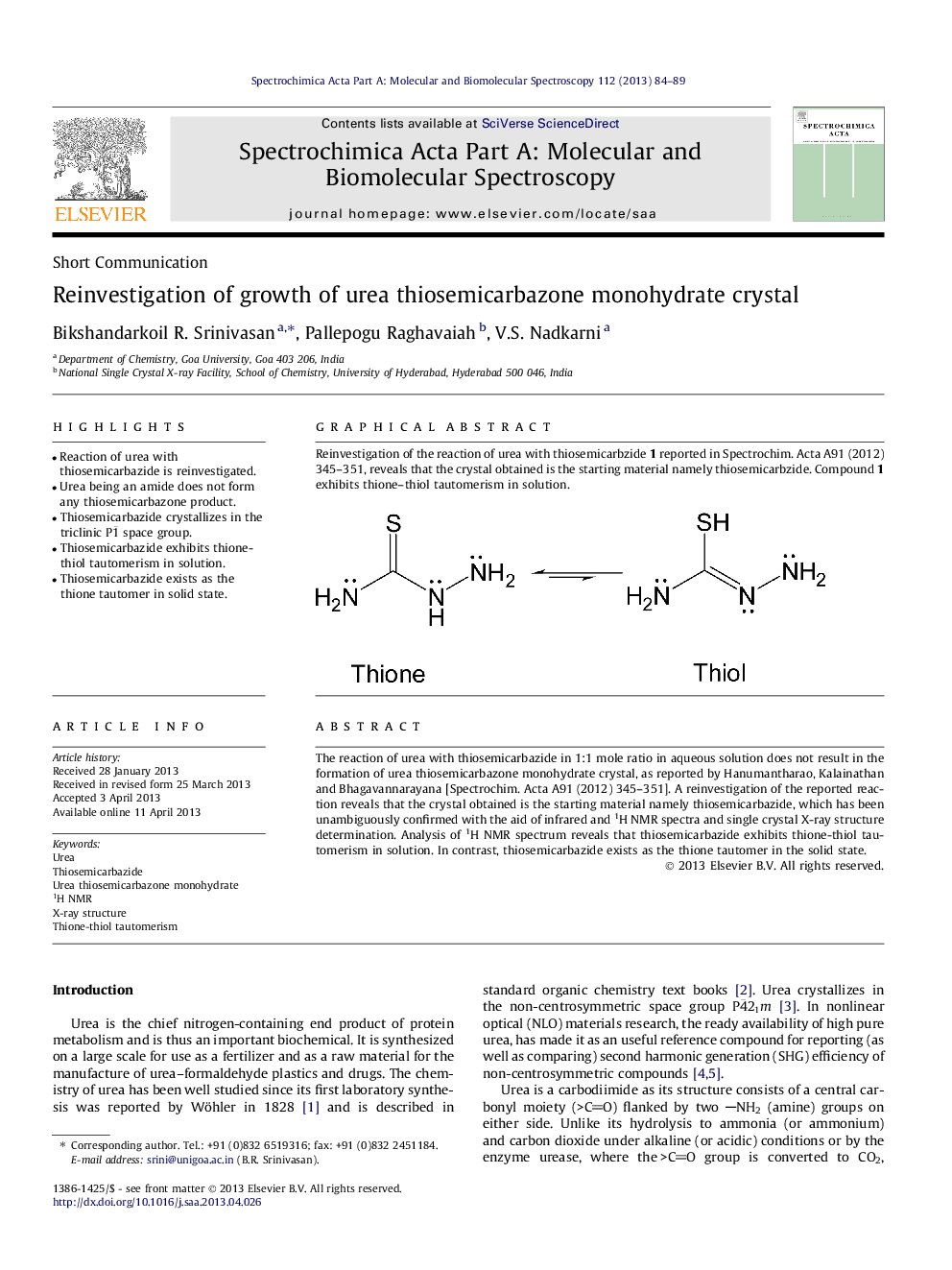
The reaction of urea with thiosemicarbazide in 1:1 mole ratio in aqueous solution does not result in the formation of urea thiosemicarbazone monohydrate crystal, as reported by Hanumantharao, Kalainathan and Bhagavannarayana [Spectrochim. Acta A91 (2012) 345–351]. A reinvestigation of the reported reaction reveals that the crystal obtained is the starting material namely thiosemicarbazide, which has been unambiguously confirmed with the aid of infrared and 1H NMR spectra and single crystal X-ray structure determination. Analysis of 1H NMR spectrum reveals that thiosemicarbazide exhibits thione-thiol tautomerism in solution. In contrast, thiosemicarbazide exists as the thione tautomer in the solid state.
Graphical abstractReinvestigation of the reaction of urea with thiosemicarbzide 1 reported in Spectrochim. Acta A91 (2012) 345–351, reveals that the crystal obtained is the starting material namely thiosemicarbzide. Compound 1 exhibits thione–thiol tautomerism in solution.Figure optionsDownload full-size imageDownload as PowerPoint slide