| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230732 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 13 Pages |
•FT-IR and FT-Raman spectra of ethyl 4-aminobenzoate (EAB) in the solid phase were recorded and analyzed.•The optimized geometry and vibrational wavenumbers were computed using DFT methods.•The complete vibrational assignment and spectroscopic analysis have been carried out.•The first order hyperpolarizability and HOMO, LUMO energy gap were theoretically predicted.•The NBO analysis explained the intramolecular hydrogen bonding.
The FT-IR and FT-Raman spectra of ethyl 4-aminobenzoate (EAB) in the solid phase were recorded. The fundamental vibrational wavenumbers, intensities of vibrational bands and the optimized geometrical parameters of the compound were evaluated using DFT (B3LYP) method with 6-311++G(d,p) basis set. The stable geometry of the compound was determined from the potential energy surface scan. Complete vibrational assignments and Natural Bond Orbital (NBO) analysis for the title compound were carried out. The assignments of the vibrational spectra were carried out with the help of normal co-ordinate analysis (NCA) following the Scaled Quantum Mechanical Force Field (SQMFF) methodology. The molecule orbital contributions were studied by using the total (TDOS), partial (PDOS), and overlap population (OPDOS) density of states. UV–visible spectrum of the compound was recorded and the electronic properties, such as HOMO and LUMO energies were performed by time-dependent DFT (TD-DFT) approach. Mulliken population analysis on atomic charges were also calculated. Besides, molecular electrostatic potential (MEP) and thermodynamic properties were performed.
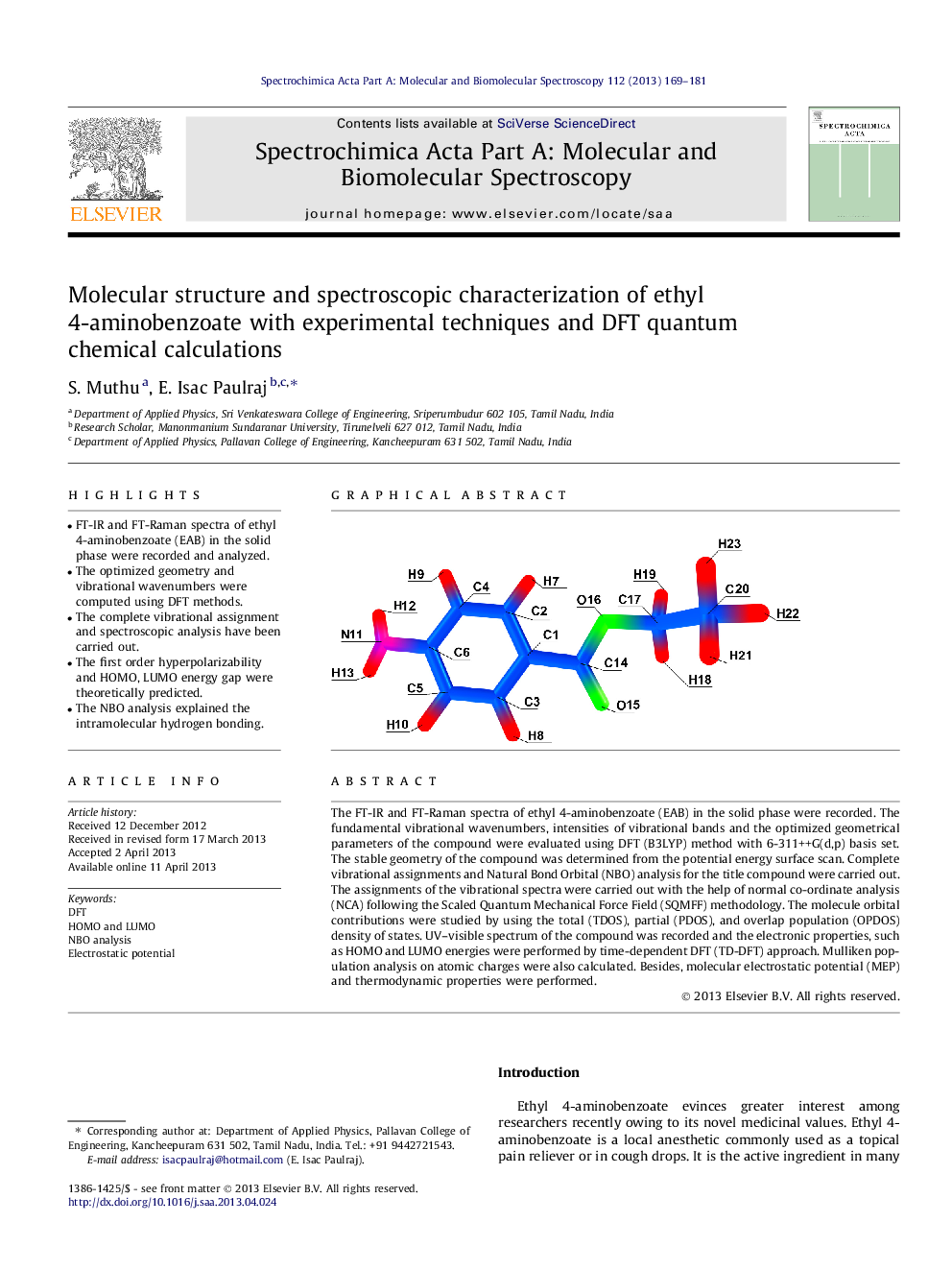
Graphical abstractA complete vibrational analysis of ethyl 4-aminobenzoate (EAB) is performed by combining the experimental and theoretical information using Pulay’s density functional theory (DFT) based on scaled quantum chemical approach. The calculated HOMO and LUMO energies show that the charge transfer occur within the molecule. Comparison of simulated spectra with the experimental spectra provides important information about the ability of the computational method to describe the vibrational modes.Figure optionsDownload full-size imageDownload as PowerPoint slide