| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230735 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 8 Pages |
•Synthesis of good DNA intercalators.•Exploring efficient chemotherapeutic agents.•Better antimicrobial active agents.•Effective DNA cleavage activators.•Excellent Cu(II) DNA binder.
Five new Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) mixed ligand complexes have been synthesized using a Schiff base precursor (obtained by the condensation of N-(4-aminophenyl)acetamide and 4-chlorobenzaldehyde) as main ligand and 1,10-phenanthroline as co-ligand. They have been characterized by microanalytical data, IR, UV–Vis, magnetic moment values, conductivity and electrochemical measurements. The spectral data reveal that all the complexes exhibit octahedral geometry. The high electrical conductance of the complexes supports their electrolytic nature. The monomeric nature of the complexes has been assessed from their magnetic susceptibility values. These complexes are better antimicrobial active agents than the free ligands. DNA (CT) binding properties of these complexes have been explored by UV–Vis., viscosity measurements, cyclic voltammetry, and differential pulse voltammetry measurements. The oxidative cleavage activity of the complexes has been studied using supercoiled pUC19 DNA by gel electrophoresis. The experimental results show that the complexes are good intercalators.
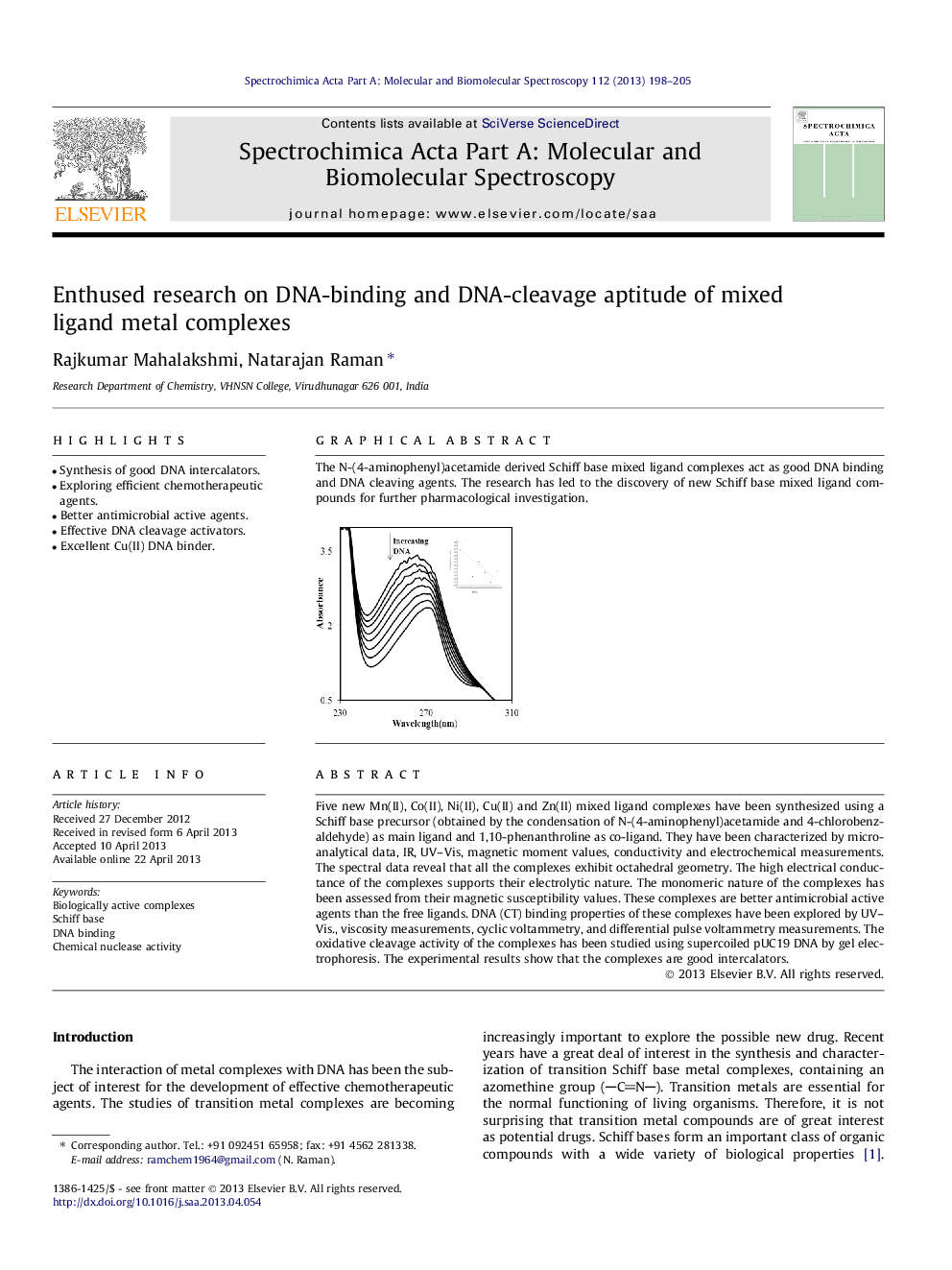
Graphical abstractThe N-(4-aminophenyl)acetamide derived Schiff base mixed ligand complexes act as good DNA binding and DNA cleaving agents. The research has led to the discovery of new Schiff base mixed ligand compounds for further pharmacological investigation.Figure optionsDownload full-size imageDownload as PowerPoint slide