| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230743 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 6 Pages |
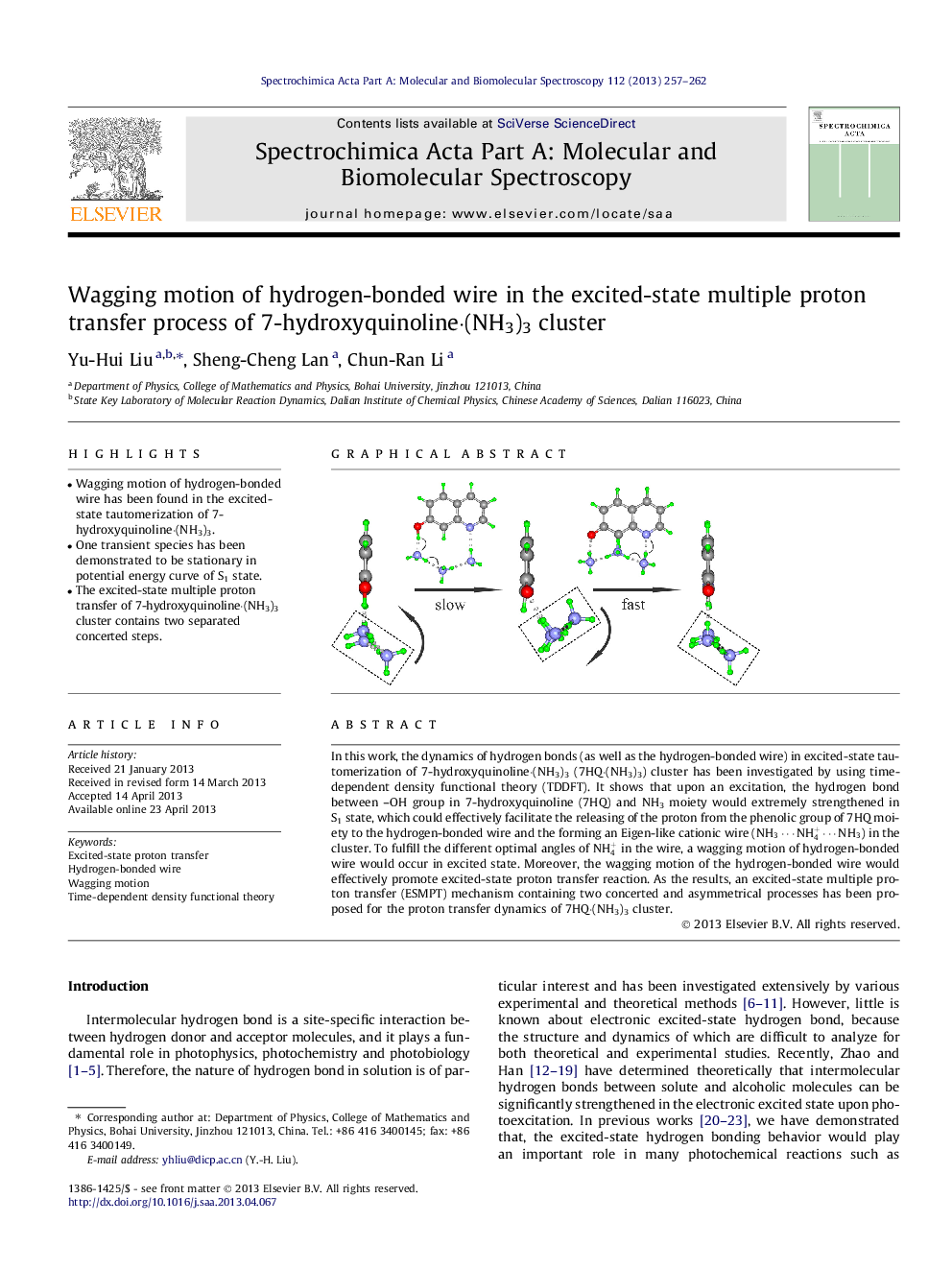
•Wagging motion of hydrogen-bonded wire has been found in the excited-state tautomerization of 7-hydroxyquinoline·(NH3)3.•One transient species has been demonstrated to be stationary in potential energy curve of S1 state.•The excited-state multiple proton transfer of 7-hydroxyquinoline·(NH3)3 cluster contains two separated concerted steps.
In this work, the dynamics of hydrogen bonds (as well as the hydrogen-bonded wire) in excited-state tautomerization of 7-hydroxyquinoline·(NH3)3 (7HQ⋅(NH3)3) cluster has been investigated by using time-dependent density functional theory (TDDFT). It shows that upon an excitation, the hydrogen bond between –OH group in 7-hydroxyquinoline (7HQ) and NH3 moiety would extremely strengthened in S1 state, which could effectively facilitate the releasing of the proton from the phenolic group of 7HQ moiety to the hydrogen-bonded wire and the forming an Eigen-like cationic wire (NH3⋯NH4+⋯NH3) in the cluster. To fulfill the different optimal angles of NH4+ in the wire, a wagging motion of hydrogen-bonded wire would occur in excited state. Moreover, the wagging motion of the hydrogen-bonded wire would effectively promote excited-state proton transfer reaction. As the results, an excited-state multiple proton transfer (ESMPT) mechanism containing two concerted and asymmetrical processes has been proposed for the proton transfer dynamics of 7HQ⋅(NH3)3 cluster.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide