| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230744 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 13 Pages |
•Basing on the DFT QCC the structures of these compounds have been proposed.•The IR and RS wavenumbers were calculated for monomers and dimers.•IR, RS and DFT methods confirm the existence of an intra- and an inter-molecular HBs.•The vibrational characteristics of the hydrazo-bond have been reported.•The influence of substitution position of the methyl group on the vibrational data.
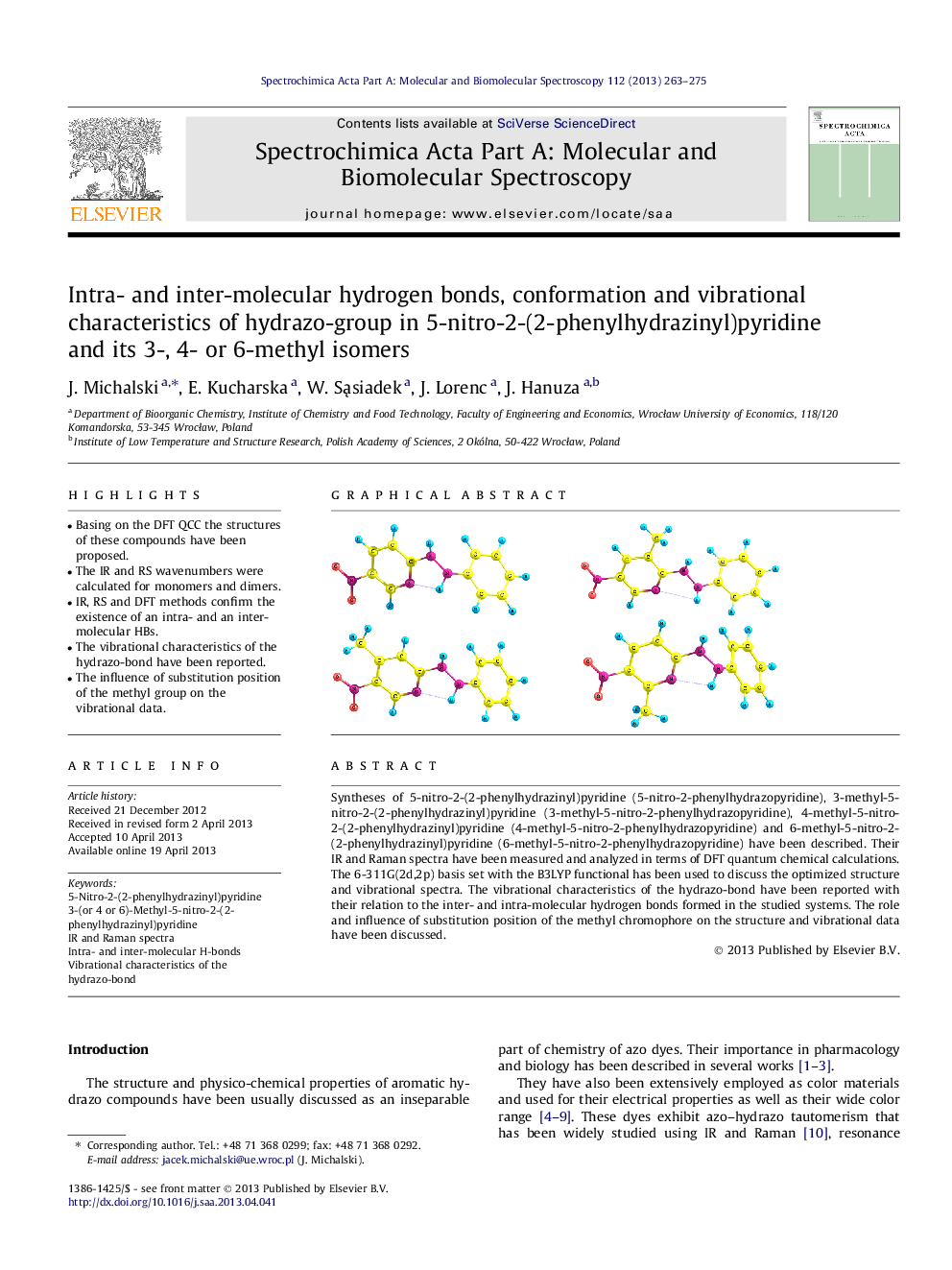
Syntheses of 5-nitro-2-(2-phenylhydrazinyl)pyridine (5-nitro-2-phenylhydrazopyridine), 3-methyl-5-nitro-2-(2-phenylhydrazinyl)pyridine (3-methyl-5-nitro-2-phenylhydrazopyridine), 4-methyl-5-nitro-2-(2-phenylhydrazinyl)pyridine (4-methyl-5-nitro-2-phenylhydrazopyridine) and 6-methyl-5-nitro-2-(2-phenylhydrazinyl)pyridine (6-methyl-5-nitro-2-phenylhydrazopyridine) have been described. Their IR and Raman spectra have been measured and analyzed in terms of DFT quantum chemical calculations. The 6-311G(2d,2p) basis set with the B3LYP functional has been used to discuss the optimized structure and vibrational spectra. The vibrational characteristics of the hydrazo-bond have been reported with their relation to the inter- and intra-molecular hydrogen bonds formed in the studied systems. The role and influence of substitution position of the methyl chromophore on the structure and vibrational data have been discussed.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide