| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230753 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 12 Pages |
•The hyperconjugative interactions and charge delocalization has been investigated.•The intramolecular charge transfer [n → π∗, π → π∗, π∗ → π∗] occurs in title compound.•The compound exhibits strong effective intra– and intermolecular charge transfer.•The compound shows large second–order nonlinearity.•The 5,6–disubstituted pyrimidine systems are effective in the design of new bioorganic molecules.
Structural and conformational, natural bond orbital (NBO) and nonlinear optical (NLO) analysis was performed, and 1H and 13C NMR chemical shifts values of 5-(2-Acetoxyethyl)-6-methylpyrimidin-2,4-dione [C9H12N2O4] in the ground state were calculated by using Density Functional Theory (DFT-B3LYP/6-311++G(d,p)) and Hartree–Fock (HF/6-311++G(d,p)) methods. The NMR data were calculated by means of the GIAO, CSGT, and IGAIM methods. In addition, the molecular frontier orbital energies, thermodynamic parameters (in the range of 200–700 K), molecular surfaces, Mulliken charges and atomic polar tensor-based charges were investigated. Besides, the analysis of all possible conformational of the title compound, a detailed potential energy curve for τ1(C8O3C10O4), τ2 (C8O3C10C11) and τ3 (C5C7C8O3) dihedral angles were performed in steps of 10° from 0° to 360°, and depicted to find the most stable form. Finally, the calculated HOMO and LUMO energies show that charge transfer occurs within the title compound.
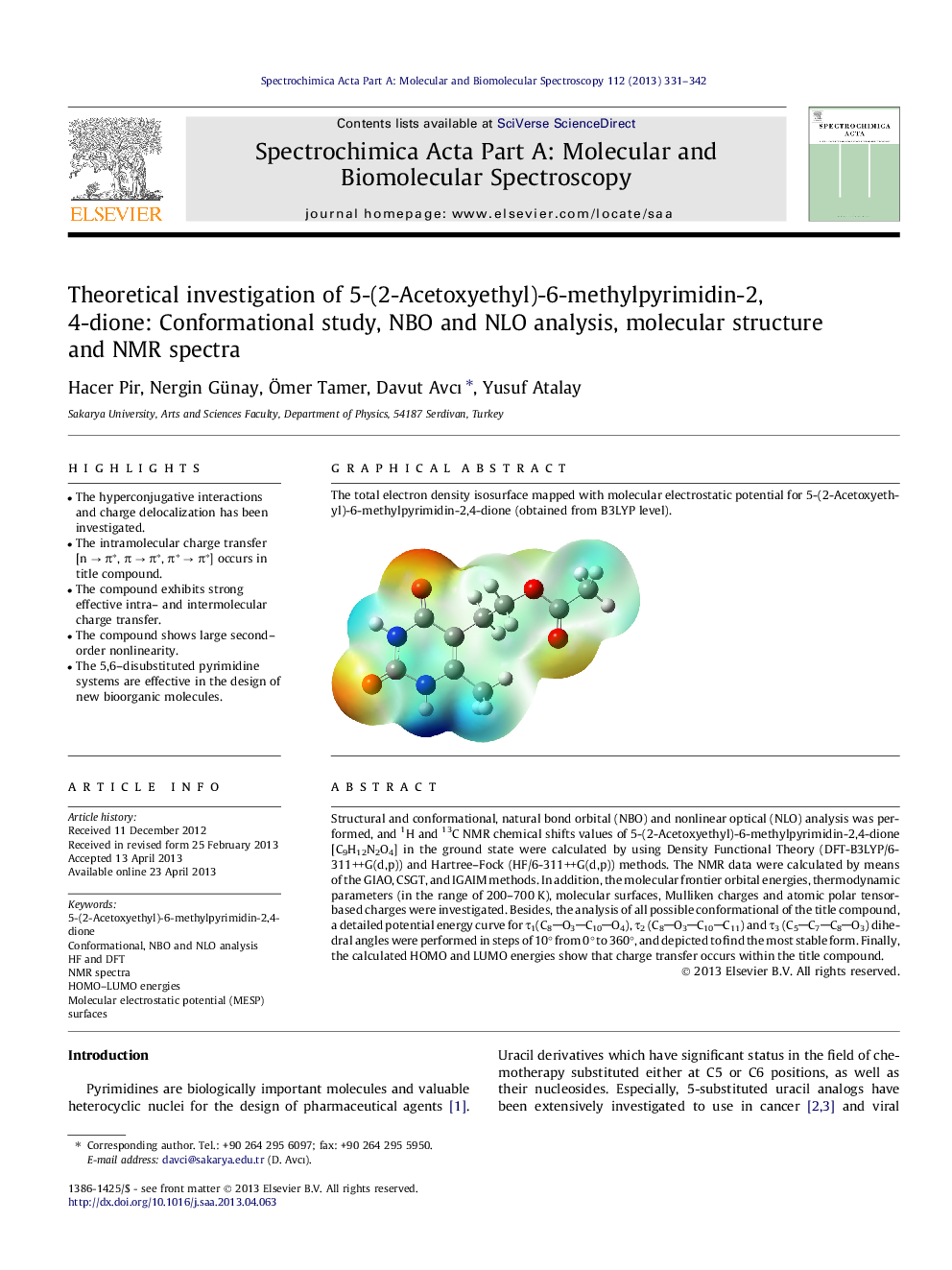
Graphical abstractThe total electron density isosurface mapped with molecular electrostatic potential for 5-(2-Acetoxyethyl)-6-methylpyrimidin-2,4-dione (obtained from B3LYP level).Figure optionsDownload full-size imageDownload as PowerPoint slide