| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1230754 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 8 Pages |
•Melaminium perchlorate mono hydrate single crystals have been synthesized.•The crystal system was identified as triclinic (P−1) and characterized by FT-IR, FT-Raman, FT-NMR studies.•Several stretching and deformation modes confirm the presence of extensive intermolecular hydrogen bonding in the crystal.•The optimized geometry and vibrational frequencies are calculated using DFT.•HOMO–LUMO energies are calculated and show that charge transfer occurs within the molecule.
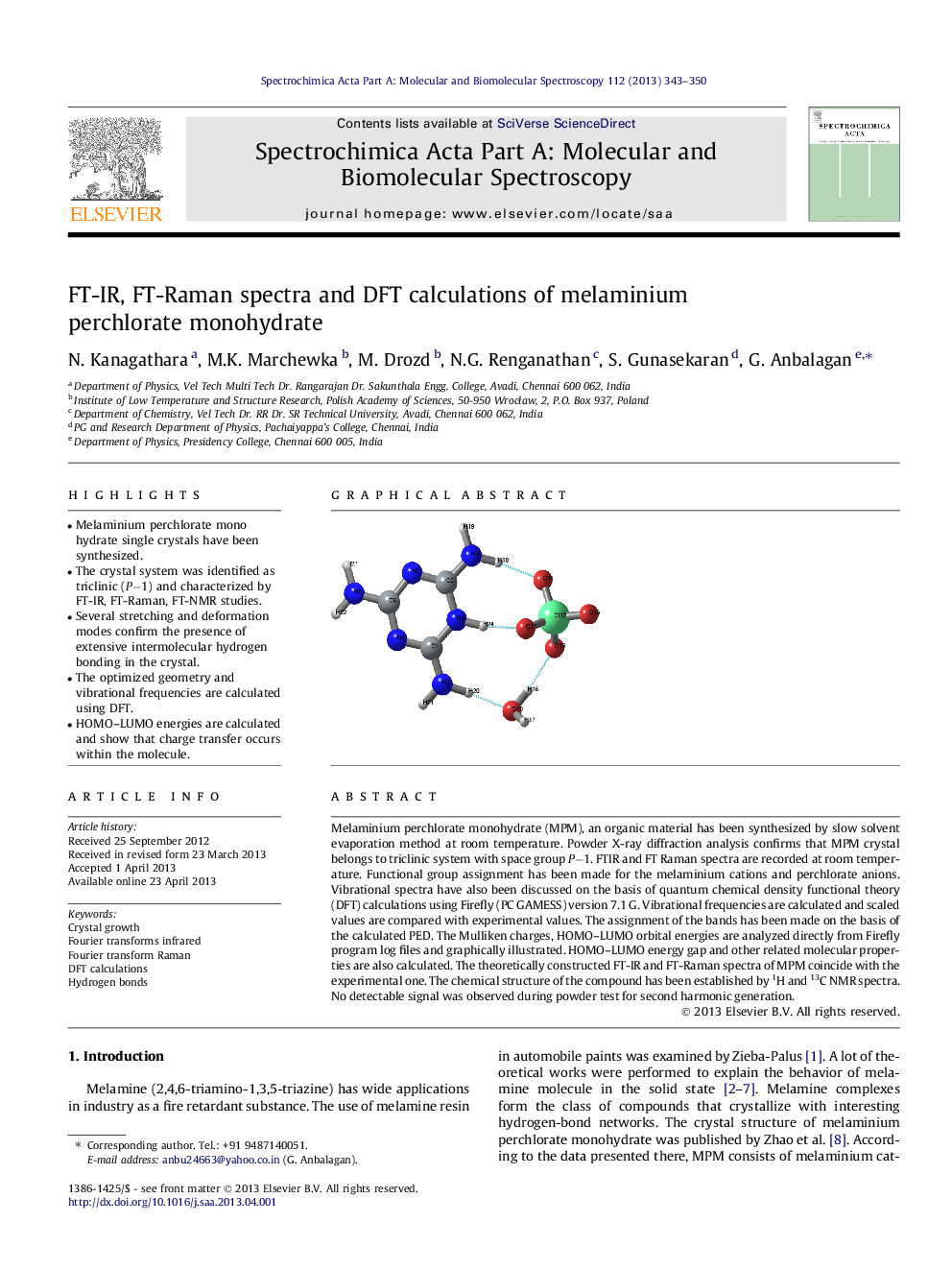
Melaminium perchlorate monohydrate (MPM), an organic material has been synthesized by slow solvent evaporation method at room temperature. Powder X-ray diffraction analysis confirms that MPM crystal belongs to triclinic system with space group P−1. FTIR and FT Raman spectra are recorded at room temperature. Functional group assignment has been made for the melaminium cations and perchlorate anions. Vibrational spectra have also been discussed on the basis of quantum chemical density functional theory (DFT) calculations using Firefly (PC GAMESS) version 7.1 G. Vibrational frequencies are calculated and scaled values are compared with experimental values. The assignment of the bands has been made on the basis of the calculated PED. The Mulliken charges, HOMO–LUMO orbital energies are analyzed directly from Firefly program log files and graphically illustrated. HOMO–LUMO energy gap and other related molecular properties are also calculated. The theoretically constructed FT-IR and FT-Raman spectra of MPM coincide with the experimental one. The chemical structure of the compound has been established by 1H and 13C NMR spectra. No detectable signal was observed during powder test for second harmonic generation.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide